Question
A rocket using hydrogen-oxygen as the fuel-oxidizer combination has a specific impulse of 360s. Calculate the ratio of propellant mass to initial mass required to
A rocket using hydrogen-oxygen as the fuel-oxidizer combination has a specific impulse of 360s. Calculate the ratio of propellant mass to initial mass required to achieve a burnout velocity equal to the escape velocity from Earth (11.19 km/s). Use the values on the table.
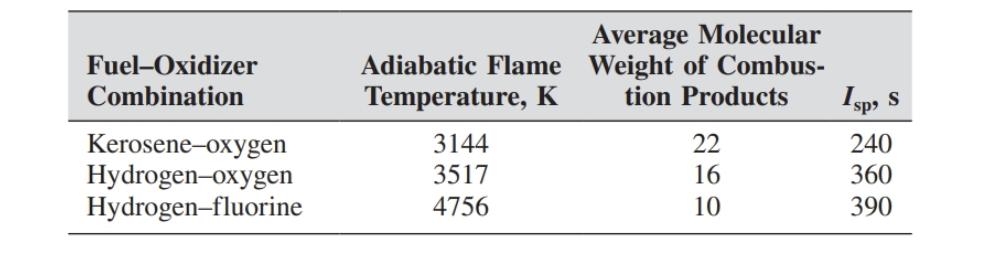
Average Molecular Fuel-Oxidizer Combination Adiabatic Flame Weight of Combus- Temperature, K Kerosene-oxygen 3144 Hydrogen-oxygen 3517 Hydrogen-fluorine 4756 tion Products Isp, S 22 260 240 16 360 10 390
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Numerical Methods For Engineers
Authors: Steven C. Chapra, Raymond P. Canale
5th Edition
978-0071244299, 0071244298
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



