Answered step by step
Verified Expert Solution
Question
1 Approved Answer
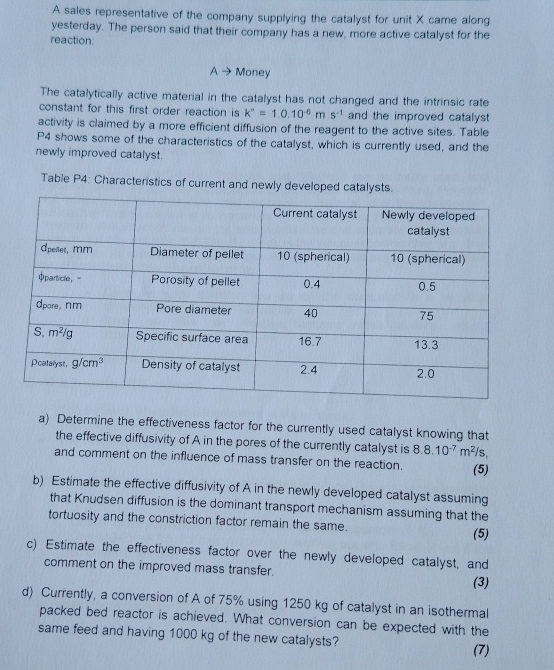
A sales representative of the company supplying the catalyst for unit x came along yesterday. The person said that their company has a new, more
A sales representative of the company supplying the catalyst for unit came along yesterday. The person said that their company has a new, more active catalyst for the reaction.
Money
The catalytically active material in the catalyst has not changed and the intrinsic rate constant for this first order reaction is and the improved catalyst activity is claimed by a more efficient diffusion of the reagent to the active sites. Table P shows some of the characteristics of the catalyst, which is currently used, and the newly improved catalyst.
Table P: Characteristics of current and newly developed catalysts.
tableCurrent catalyst,tableNewly developedcatalystDiameter of pellet,sphericalsphericalPorosity of pellet,Pore diameter,Specific surface area,Density of catalyst,
a Determine the effectiveness factor for the currently used catalyst knowing that the effective diffusivity of in the pores of the currently catalyst is and comment on the influence of mass transfer on the reaction.
b Estimate the effective diffusivity of in the newly developed catalyst assuming that Knudsen diffusion is the dominant transport mechanism assuming that the tortuosity and the constriction factor remain the same.
c Estimate the effectiveness factor over the newly developed catalyst, and comment on the improved mass transfer.
d Currently, a conversion of A of using of catalyst in an isothermal packed bed reactor is achieved. What conversion can be expected with the same feed and having of the new catalysts?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


