Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A sample of 1.00 mole of a diatomic ideal gas is initially at temperature 265 K and volume 0.200 m3. The gas first undergoes
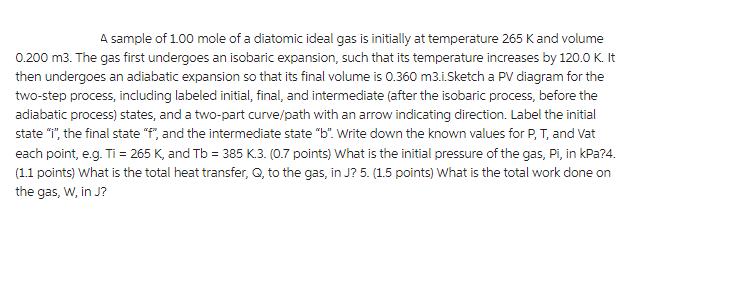
A sample of 1.00 mole of a diatomic ideal gas is initially at temperature 265 K and volume 0.200 m3. The gas first undergoes an isobaric expansion, such that its temperature increases by 120.0 K. It then undergoes an adiabatic expansion so that its final volume is 0.360 m3.i.Sketch a PV diagram for the two-step process, including labeled initial, final, and intermediate (after the isobaric process, before the adiabatic process) states, and a two-part curve/path with an arrow indicating direction. Label the initial state "i", the final state "f", and the intermediate state "b". Write down the known values for P, T, and Vat each point, e.g. Ti = 265 K, and Tb = 385 K.3. (0.7 points) What is the initial pressure of the gas, Pi, in kPa?4. (1.1 points) What is the total heat transfer, Q, to the gas, in J? 5. (1.5 points) What is the total work done on the gas, W, in J?
Step by Step Solution
★★★★★
3.49 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Grass Undergoes three states 1 Initial 2 2 Intermediate b 3 final f E Pi Vi Ti iso baric Pb Vb Tb St...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


