Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A sample of diatomic ideal gas is initially at state A with pressure PA = 1.21 atm, volume VA = 1.14 m, and temperature
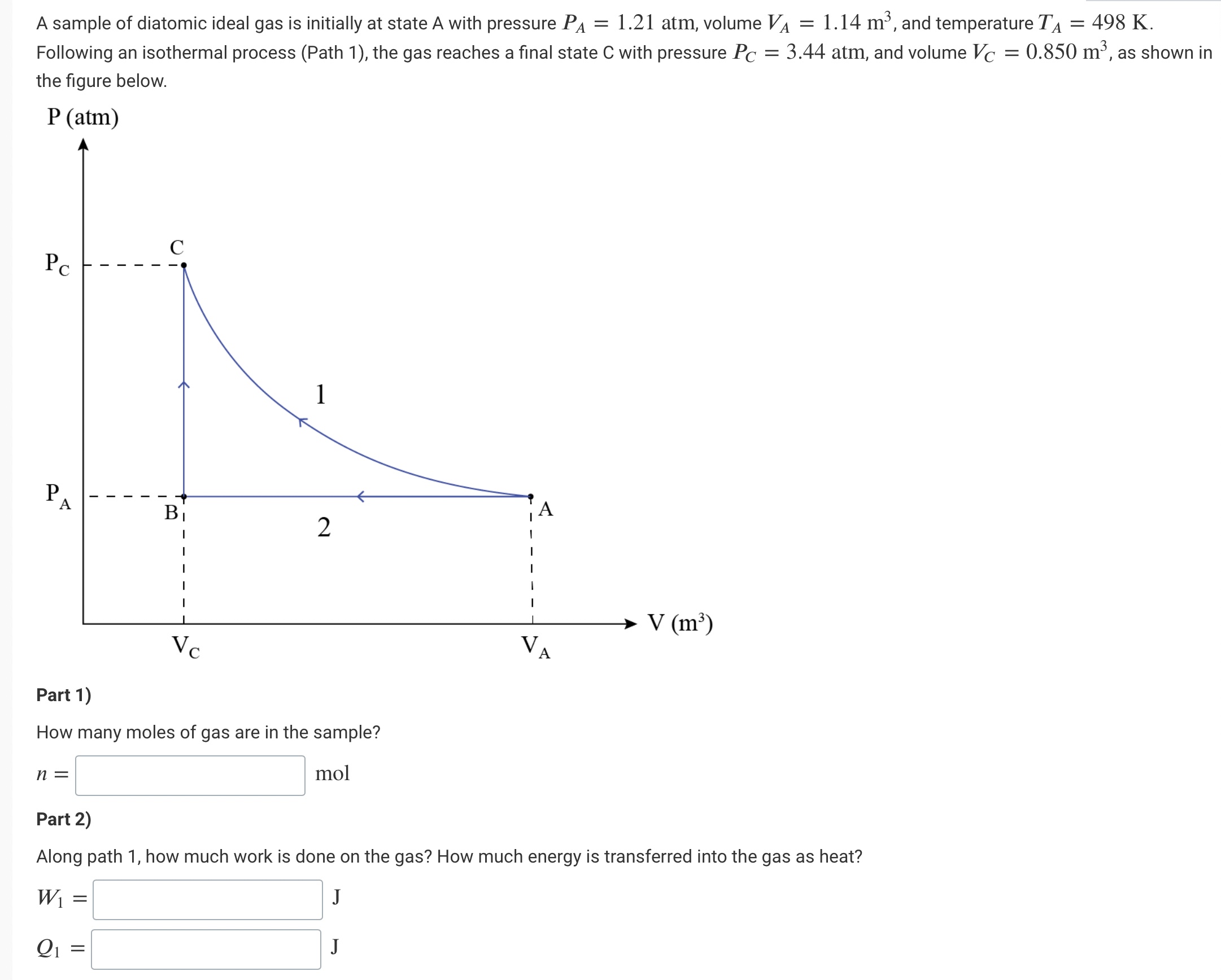

A sample of diatomic ideal gas is initially at state A with pressure PA = 1.21 atm, volume VA = 1.14 m, and temperature T = 498 K. Following an isothermal process (Path 1), the gas reaches a final state C with pressure Pc = 3.44 atm, and volume Vc = 0.850 m, as shown in the figure below. P (atm) PC C P A 1 A B 2 V (m) VA Part 1) Vc How many moles of gas are in the sample? n = mol Part 2) Along path 1, how much work is done on the gas? How much energy is transferred into the gas as heat? W = Q1 J J Part 3) Starting at the same initial state A, an identical sample of ideal gas now follows path 2 with an isobaric process from state A to B and an isovolumetric process from state B to C. How much energy is transferred to the gas as heat along path 2? J Q2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


