Question: A schematic for a hydrogen atom is shown in the figure below. In a hydrogen atom, an electron orbits a proton. The distance between
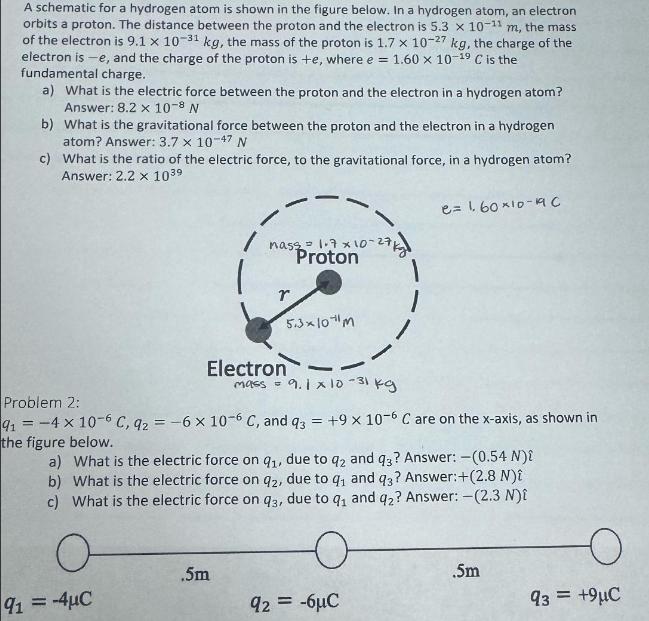
A schematic for a hydrogen atom is shown in the figure below. In a hydrogen atom, an electron orbits a proton. The distance between the proton and the electron is 5.3 x 10-11 m, the mass of the electron is 9.1 x 10-31 kg, the mass of the proton is 1.7 x 10-27 kg, the charge of the electron is -e, and the charge of the proton is +e, where e = 1.60 x 10- C is the fundamental charge. a) What is the electric force between the proton and the electron in a hydrogen atom? Answer: 8.2 x 10-8 N b) What is the gravitational force between the proton and the electron in a hydrogen atom? Answer: 3.7 10-47 N c) What is the ratio of the electric force, to the gravitational force, in a hydrogen atom? Answer: 2.2 x 103 91-4C nassa 1.7 x 10-27/ Proton r .5m 5.310 M Electron - mass = 9.1 x10-31 kg Problem 2: 91 = 4 x 10-6 C, 92 = -6 x 10-6 C, and q3 = +9 x 10-6 C are on the x-axis, as shown in the figure below. a) What is the electric force on q1, due to q2 and q3? Answer: -(0.54 N){ b) What is the electric force on q2, due to q and q3? Answer: +(2.8 N) c) What is the electric force on q3, due to q and q2? Answer: -(2.3 N){ O 1 92 = -6 e=16010-190 .5m o 93 = +9C
Step by Step Solution
3.41 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


