Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The figure shown below is a phase diagram of the Lithium-Antimony binary system. According to the phase diagram, possible chemical reactions between Sb and
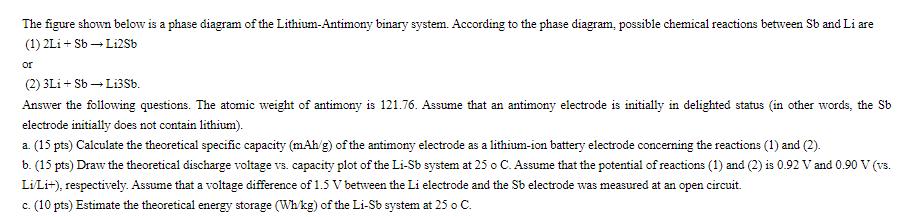
The figure shown below is a phase diagram of the Lithium-Antimony binary system. According to the phase diagram, possible chemical reactions between Sb and Li are (1) 2Li+Sb Li2Sb or (2) 3Li + Sb Li3Sb. Answer the following questions. The atomic weight of antimony is 121.76. Assume that an antimony electrode is initially in delighted status (in other words, the Sb electrode initially does not contain lithium). a. (15 pts) Calculate the theoretical specific capacity (mAh/g) of the antimony electrode as a lithium-ion battery electrode concerning the reactions (1) and (2). b. (15 pts) Draw the theoretical discharge voltage vs. capacity plot of the Li-Sb system at 25 o C. Assume that the potential of reactions (1) and (2) is 0.92 V and 0.90 V (vs. Li/Li+), respectively. Assume that a voltage difference of 1.5 V between the Li electrode and the Sb electrode was measured at an open circuit. c. (10 pts) Estimate the theoretical energy storage (Wh/kg) of the Li-Sb system at 25 o C.
Step by Step Solution
★★★★★
3.33 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
a For reaction 1 2Li Sb Li2Sb the theoretical specific capacity of the antimony electrode is calculated as follows The molar mass of Sb is 12176 gmol ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


