Question
A sealed piston-cylinder assembly initially contains a gas at temperature T, pressure P , and volume . The piston has a mass m, a
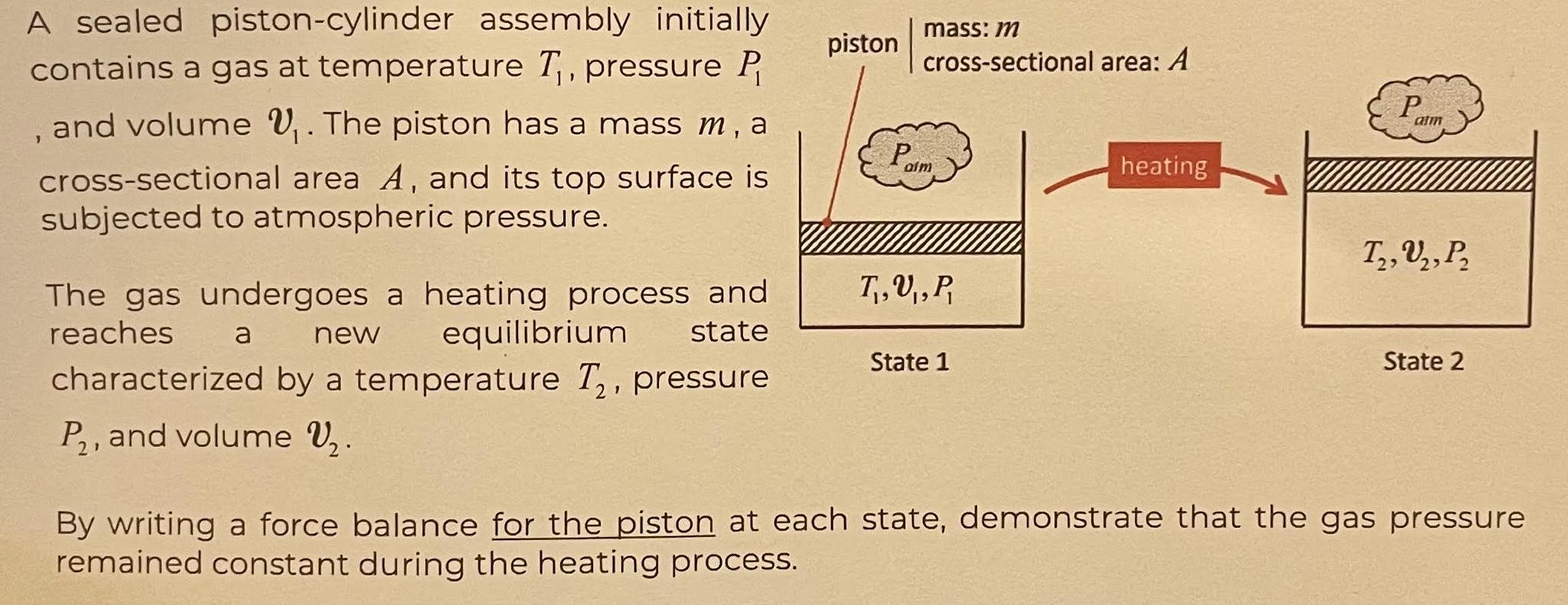
A sealed piston-cylinder assembly initially contains a gas at temperature T, pressure P , and volume . The piston has a mass m, a cross-sectional area A, and its top surface is subjected to atmospheric pressure. The gas undergoes a heating process and reaches equilibrium state a new characterized by a temperature T, pressure P, and volume V. piston mass: m cross-sectional area: A Paim T,U, P, State 1 heating P atm T, U, P 2' State 2 By writing a force balance for the piston at each state, demonstrate that the gas pressure remained constant during the heating process.
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
Lets start by writing the force balance for the piston in State 1 Ftop Fbottom Fatmos where Ftop is ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



