Answered step by step
Verified Expert Solution
Question
1 Approved Answer
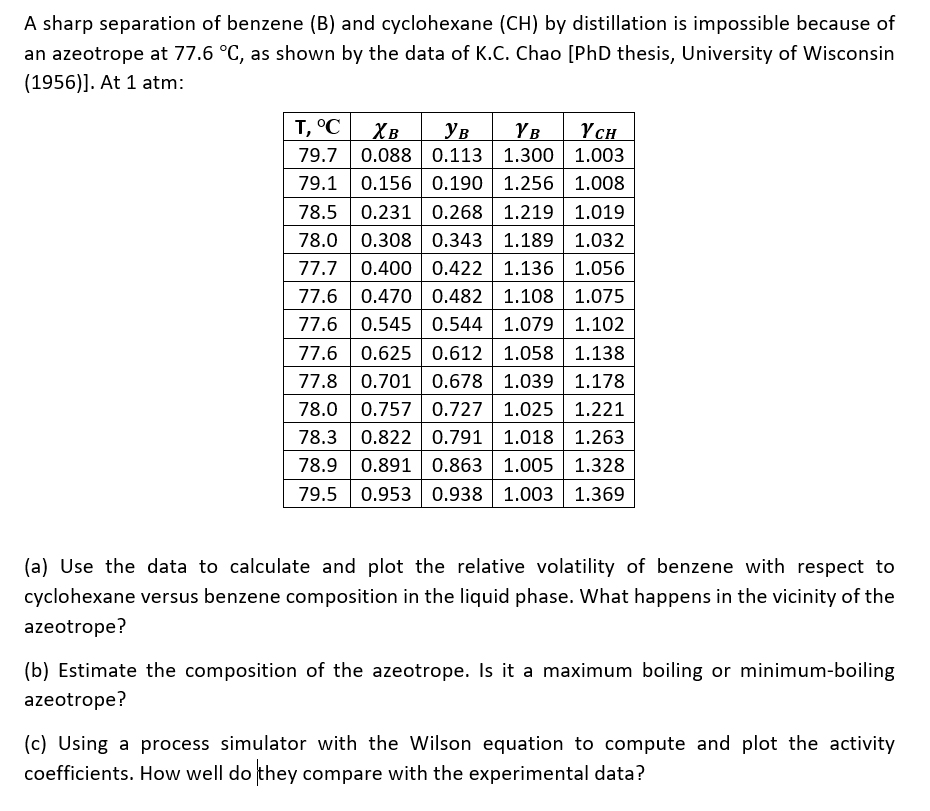
A sharp separation of benzene ( B ) and cyclohexane ( C H ) by distillation is impossible because of an azeotrope at 7 7
A sharp separation of benzene and cyclohexane by distillation is impossible because of
an azeotrope at as shown by the data of KC Chao PhD thesis, University of Wisconsin
At atm:
a Use the data to calculate and plot the relative volatility of benzene with respect to
cyclohexane versus benzene composition in the liquid phase. What happens in the vicinity of the
azeotrope?
b Estimate the composition of the azeotrope. Is it a maximum boiling or minimumboiling
azeotrope?
c Using a process simulator with the Wilson equation to compute and plot the activity
coefficients. How well do they compare with the experimental data?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


