Question
Mr. Knight still can't find some of his enthalpy values. The last equation on his list is this: CHC130) + 3HCl(g) CH4(g) + 3Cl2(g)
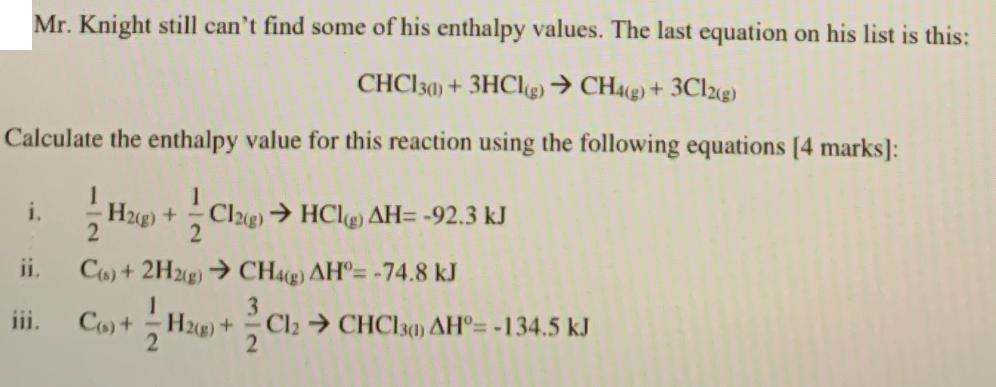
Mr. Knight still can't find some of his enthalpy values. The last equation on his list is this: CHC130) + 3HCl(g) CH4(g) + 3Cl2(g) Calculate the enthalpy value for this reaction using the following equations [4 marks]: 1 i. H2(g) + Cl2(g) HCl(g) AH= -92.3 kJ ii. C(s) + 2H2(g) CH4(g) AH= -74.8 kJ iii. C(s)+ H2(g) + Cl2 CHCl30) AH= -134.5 kJ 2
Step by Step Solution
3.60 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
I calculche we enthalpy Change por tu given by manipulating he provided Equations 2 CHC13 1 3HC g ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: Alan Giambattista, Betty Richardson, Robert Richardson
2nd edition
77339681, 978-0077339685
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



