Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A simple non-ideal model of a gas takes into account the interactions of the gas molecules, and their finite volume. Consider one such modification
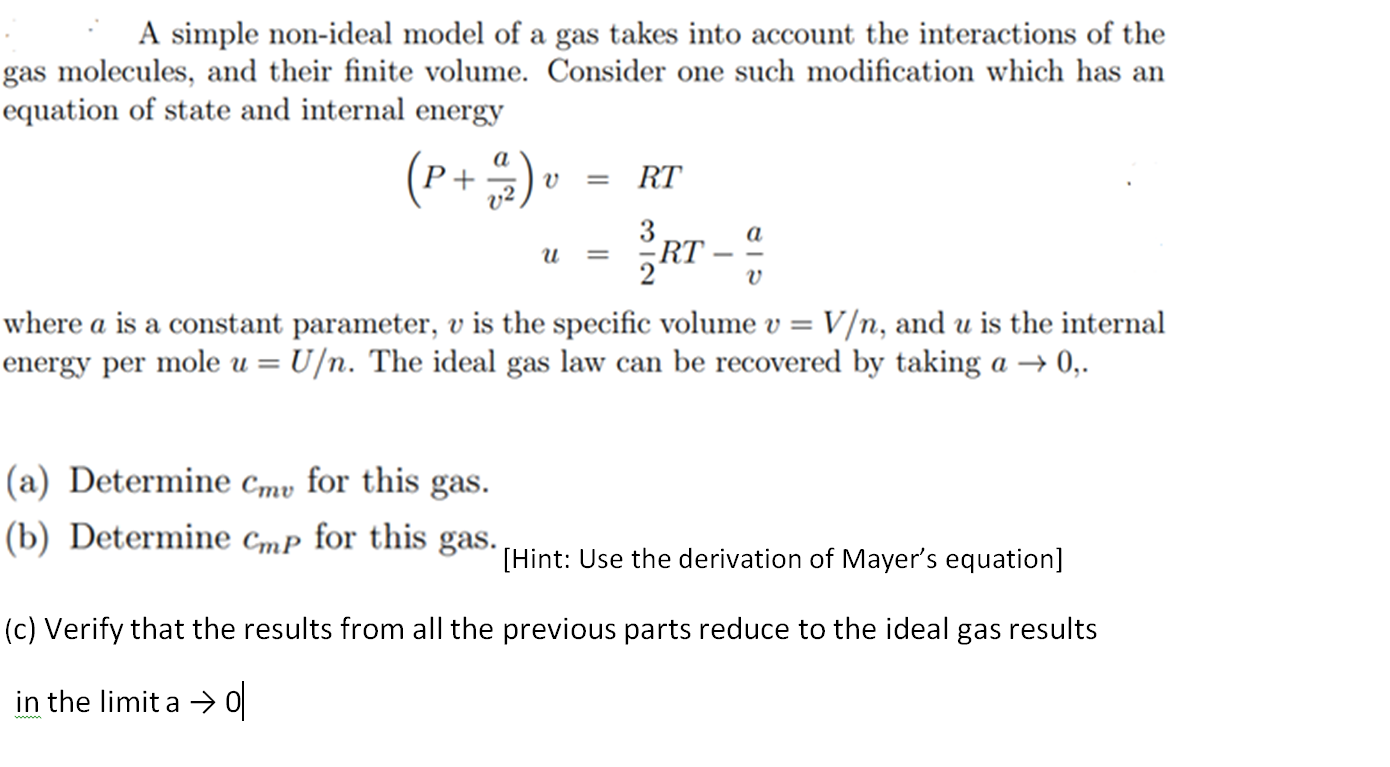
A simple non-ideal model of a gas takes into account the interactions of the gas molecules, and their finite volume. Consider one such modification which has an equation of state and internal energy (P + 2) v = RT u == IRT - a where a is a constant parameter, v is the specific volume v = V/n, and u is the internal energy per mole u = U/n. The ideal gas law can be recovered by taking a 0,. (a) Determine Cmv for this gas. (b) Determine Cmp for this gas. [Hint: Use the derivation of Mayer's equation] (c) Verify that the results from all the previous parts reduce to the ideal gas results in the limit a 0
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


