Question
A small reaction bomb fitted with a sensitive pressure measuring device is flushed out and filled with pure dimethyl ether at 140'C and 760
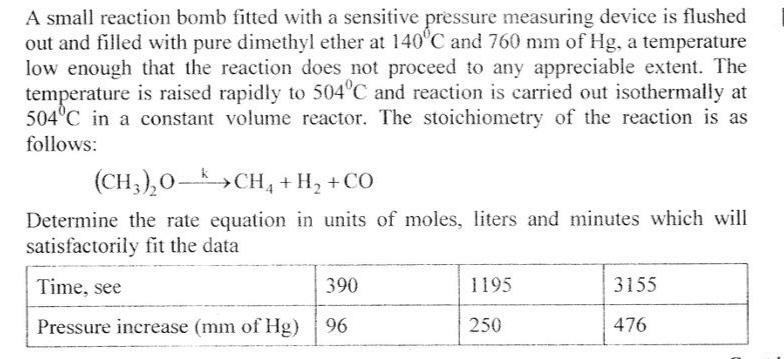
A small reaction bomb fitted with a sensitive pressure measuring device is flushed out and filled with pure dimethyl ether at 140'C and 760 mm of Hg, a temperature low enough that the reaction does not proceed to any appreciable extent. The temperature is raised rapidly to 504C and reaction is carried out isothermally at 504C in a constant volume reactor. The stoichiometry of the reaction is as follows: (CH,),0- (CH,),0->CH, + H, +CO Determine the rate equation in units of moles, liters and minutes which will satisfactorily fit the data 390 1195 3155 Time, see Pressure increase (mm of Hg) 476 96 250
Step by Step Solution
3.30 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Physics
Authors: Jearl Walker, Halliday Resnick
10th Extended edition
978-1118230718, 111823071X, 978-1118230725
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



