Question
The equilibrium reaction NO(g) 2NO2(g) has been thoroughly studied. If the total pressure in a flask containing NO, and N2O4 gas at 25 C
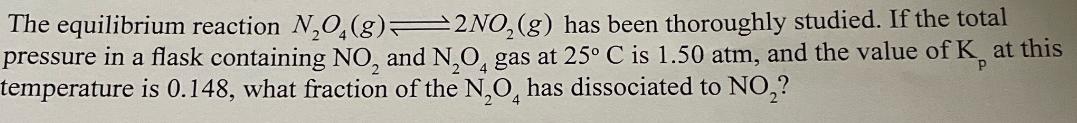
The equilibrium reaction NO(g) 2NO2(g) has been thoroughly studied. If the total pressure in a flask containing NO, and N2O4 gas at 25 C is 1.50 atm, and the value of K at this temperature is 0.148, what fraction of the N,O has dissociated to NO?
Step by Step Solution
3.38 Rating (173 Votes )
There are 3 Steps involved in it
Step: 1
To solve this problem we can use the equilibrium expression and the given value of Kp to find the fr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials Of Organizational Behavior Bridging Science And Practice
Authors: Talya Bauer, Berrin Erdogan
1st Edition
1453339248, 978-1453339244
Students also viewed these Law questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



