Answered step by step
Verified Expert Solution
Question
1 Approved Answer
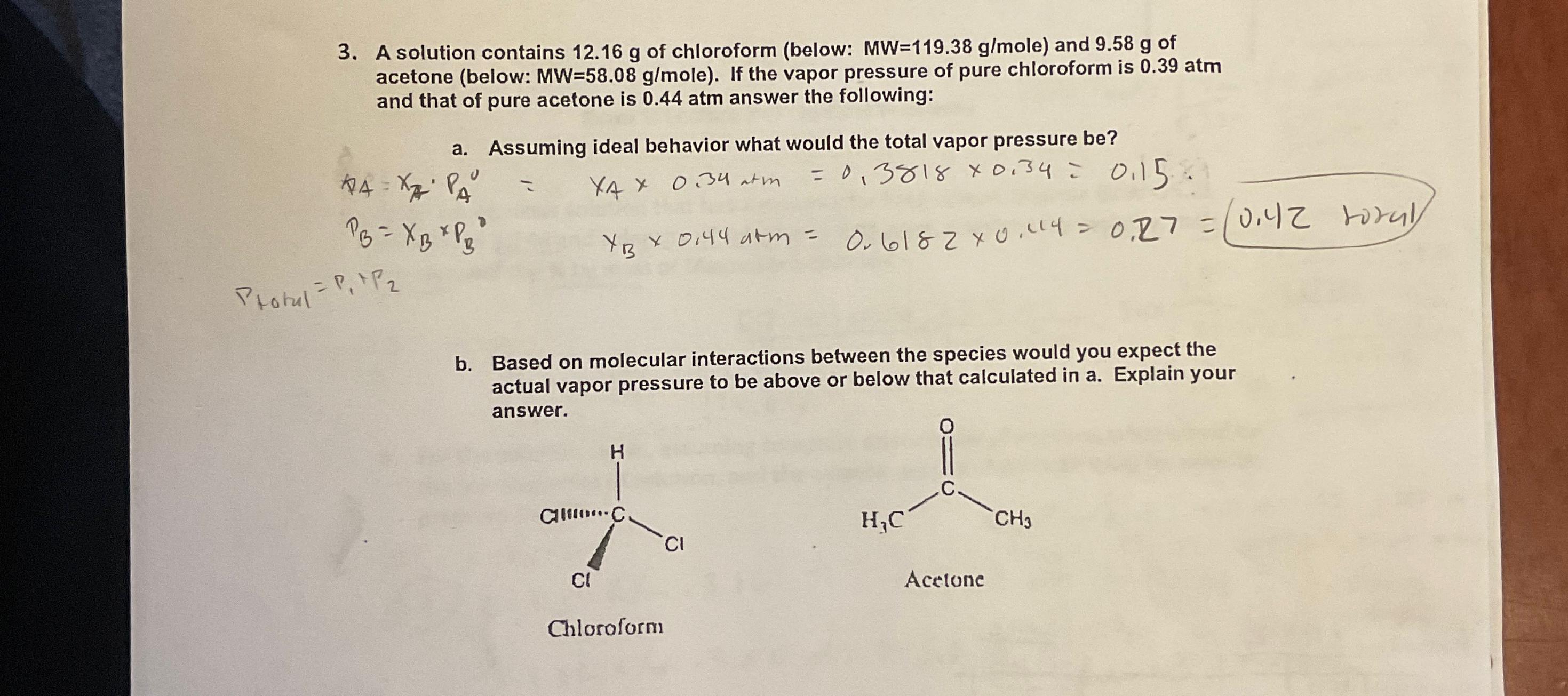
A solution contains 12.16g of chloroform (below: MW=119.38(g)/(m)ole ) and 9.58g of acetone (below: MW=58.08(g)/(m)ole ). If the vapor pressure of pure chloroform is 0.39atm
A solution contains
12.16gof chloroform (below:
MW=119.38(g)/(m)ole) and
9.58gof acetone (below:
MW=58.08(g)/(m)ole). If the vapor pressure of pure chloroform is
0.39atmand that of pure acetone is
0.44atmanswer the following:\ a. Assuming ideal behavior what would the total vapor pressure be?\
x_(A)=x_(A)*P_(A)^(0)=x_(A)\\\\times 0.34atm=0.3818\\\\times 0.34=0.15\ P_(B)=x_(B)\\\\times P_(B)^(0)x_(B)\\\\times 0.44atm=0.6182\\\\times 0.14=0.27=0.42 toral \ P_(totul )=P_(1)+P_(2)\ b. Based on molecular interactions between the species would you expect the actual vapor pressure to be above or below that calculated in a. Explain your answer.\ Acelone\ Chloratorm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


