Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A solution is made by dissolving 0.558 mol of nonelectrolyte solute in 779 g of benzene. Calculate the freezing point, T, and boiling point,
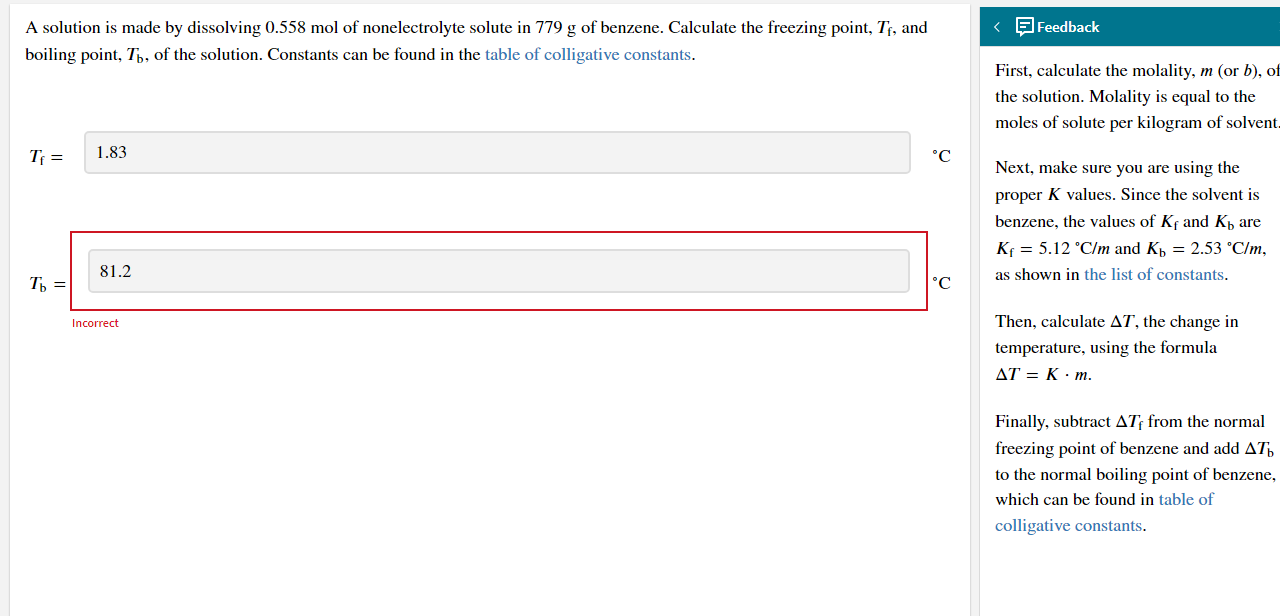
A solution is made by dissolving 0.558 mol of nonelectrolyte solute in 779 g of benzene. Calculate the freezing point, T, and boiling point, Tb, of the solution. Constants can be found in the table of colligative constants. T = 1.83 = 81.2 Incorrect Feedback First, calculate the molality, m (or b), of the solution. Molality is equal to the moles of solute per kilogram of solvent. C Next, make sure you are using the C proper K values. Since the solvent is benzene, the values of Kf and K are K = 5.12 C/m and K = 2.53 C/m, as shown in the list of constants. Then, calculate AT, the change in temperature, using the formula AT = K.m. Finally, subtract AT from the normal freezing point of benzene and add A to the normal boiling point of benzene, which can be found in table of colligative constants.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


