Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A solution made with 0.134 moles of a triprotic acid (HA, K1=6.54x10-2; K2=6.12x10-5; Kas 7.40108) dissolved in 162 mL of solution, was titrated with
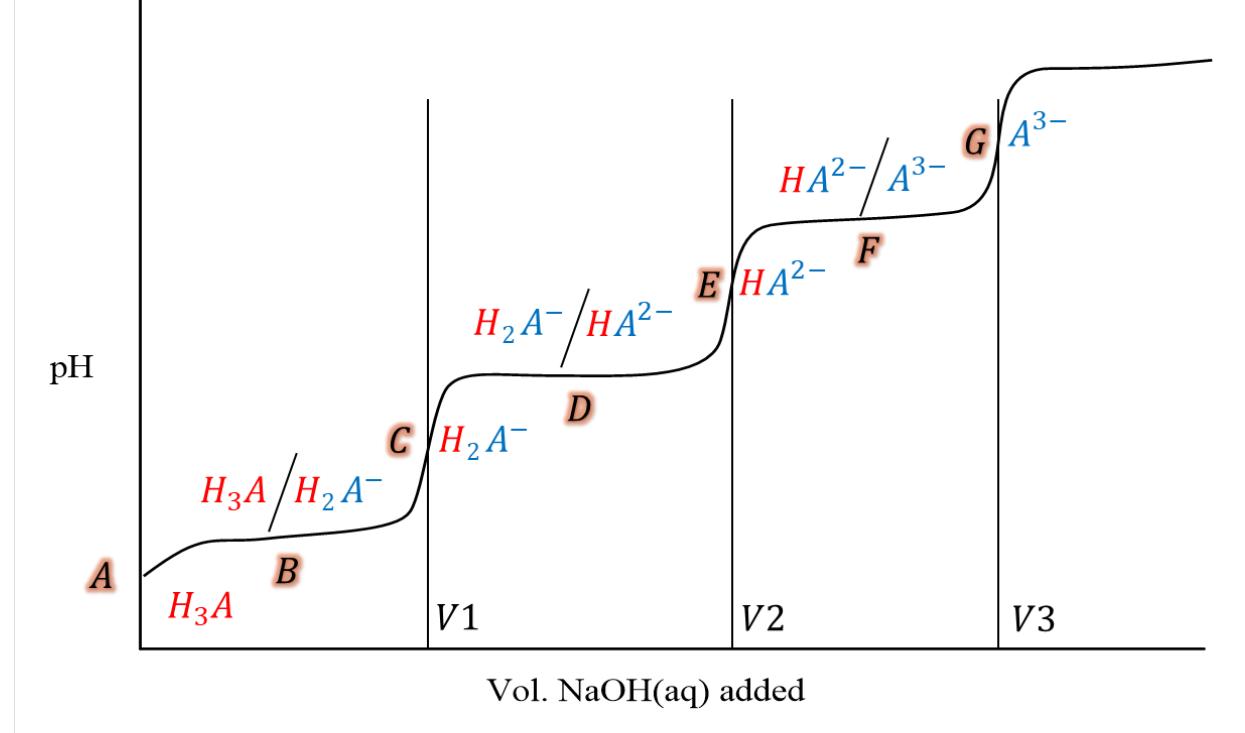
A solution made with 0.134 moles of a triprotic acid (HA, K1=6.54x10-2; K2=6.12x10-5; Kas 7.40108) dissolved in 162 mL of solution, was titrated with 0.807 M NaOH. Answer the following question about the pH of the solution, during this titration experiment: (Points A-G correspond to initial, half-way and equivalence points throughout the titration.) 3) What volume of 0.807 M NaOH solution do you need to add, to get to the final equivalence point of this titration? Volume of base (mL) = number (rtol=0.03, atol=1e-08) 4) What is the pH at equivalent point G? = number (rtol=0.03, atol=1e-08) pH A HA/HA B HA HA-/HA- D CHA V1 HA- E HA- 2-//43 F V2 Vol. NaOH(aq) added GA- V3
Step by Step Solution
★★★★★
3.30 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Given For Triprotic Acid Ka1654102 Ka2612105 Ka375108 noofmolesofAcid0134 Volumeofacid162...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


