Question
A solution of methyl acetate (C3HO) and water is boiling at 99.5 C. A sample of the vapor above the solution is cooled until
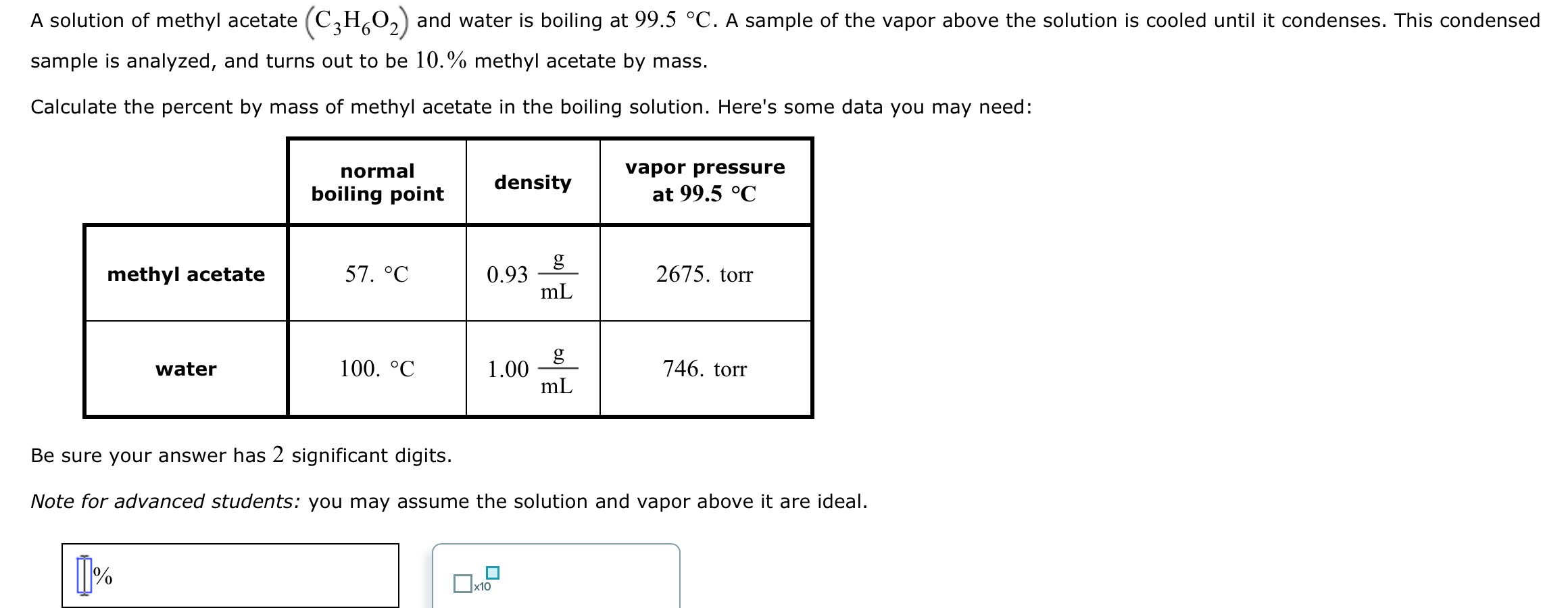
A solution of methyl acetate (C3HO) and water is boiling at 99.5 C. A sample of the vapor above the solution is cooled until it condenses. This condensed sample is analyzed, and turns out to be 10.% methyl acetate by mass. Calculate the percent by mass of methyl acetate in the boiling solution. Here's some data you may need: methyl acetate water 1% normal boiling point 57. C 100. C density 0.93 1.00 x10 g mL g mL vapor pressure at 99.5 C Be sure your answer has 2 significant digits. Note for advanced students: you may assume the solution and vapor above it are ideal. 2675. torr 746. torr
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 Solution boils when the vapor pressure of the solution equals to the atmospheric pr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



