Answered step by step
Verified Expert Solution
Question
1 Approved Answer
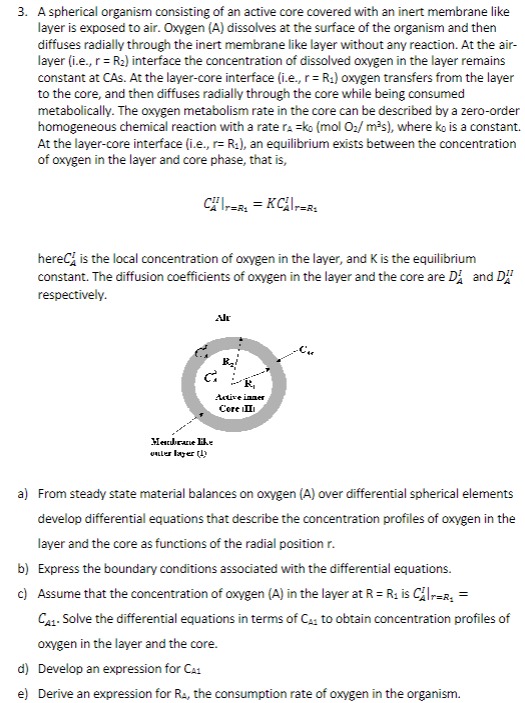
A spherical organism consisting of an active core covered with an inert membrane like layer is exposed to air. Oxygen ( A ) dissolves at
A spherical organism consisting of an active core covered with an inert membrane like layer is exposed to air. Oxygen A dissolves at the surface of the organism and then diffuses radially through the inert membrane like layer without any reaction. At the airlayer ie interface the concentration of dissolved oxygen in the layer remains constant at CAs. At the layercore interface ie oxygen transfers from the layer to the core, and then diffuses radially through the core while being consumed metabolically. The oxygen metabolism rate in the core can be described by a zeroorder
homogeneous chemical reaction with a rate where is a constant. At the layercore interface ie an equilibrium exists between the concentration of oxygen in the layer and core phase, that is here is the local concentration of oxygen in the layer, and is the equilibrium constant. The diffusion coefficients of oxygen in the layer and the core are and respectively.
a From steady state material balances on oxygen A over differential spherical elements develop differential equations that describe the concentration profiles of oxygen in the layer and the core as functions of the radial position
b Express the boundary conditions associated with the differential equations.
c Assume that the concentration of oxygen A in the layer at is Solve the differential equations in terms of to obtain concentration profiles of oxygen in the layer and the core.
d Develop an expression for
e Derive an expression for the consumption rate of oxygen in the organism.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


