Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A ) Starting with the 1 st Law, show that T T U S T P V V . ( Don t overthink this one!
A Starting with the st Law, show that
T T
U S T P
V V
Dont overthink this one!
B Using an appropriate Maxwell relation, show that
T V
U P T P
V T
C The van der Waals equation for a nonideal gas is
n P a V nb nRT
V
where a and b
are constants. Find the change in internal energy when a nonideal gas expands isothermally from initial
volume Vi to final volume Vf
D Based on your answer in C provide a physical meaning for the constant aA Starting with the Law, show that Dont overthink this one!
B Using an appropriate Maxwell relation, show that
C The van der Waals equation for a nonideal gas is where a and
are constants. Find the change in internal energy when a nonideal gas expands isothermally from initial
volume to final volume
D Based on your answer in C provide a physical meaning for the constant
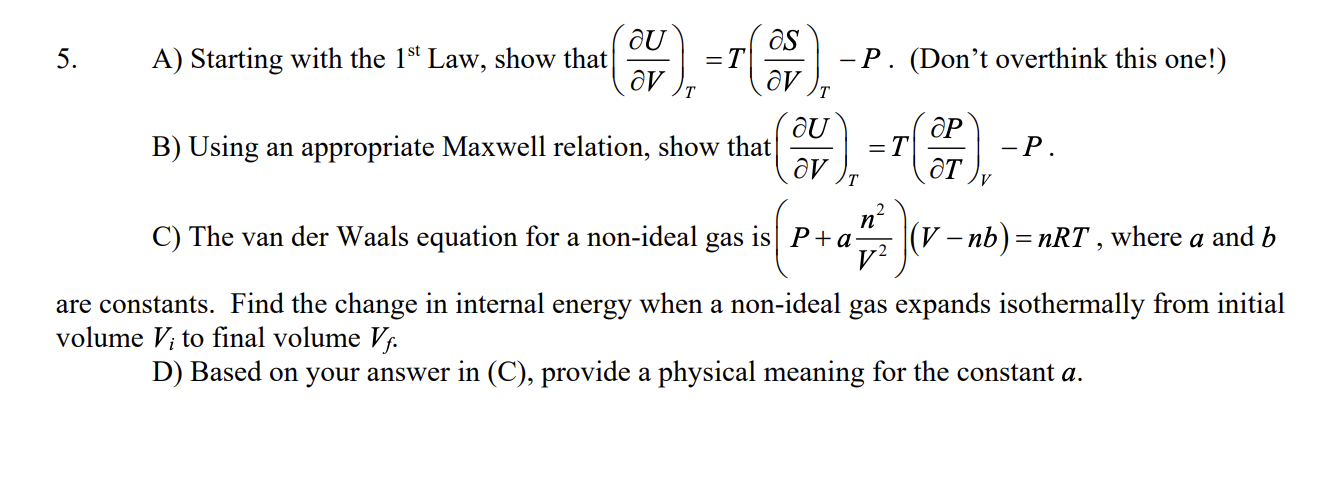
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


