Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A stoker boiler heats liquid water at 200C to superheated steam at 4 MPa and 400C. Wood fuel which has an ultimate analysis (by
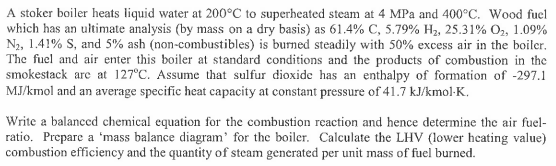
A stoker boiler heats liquid water at 200C to superheated steam at 4 MPa and 400C. Wood fuel which has an ultimate analysis (by mass on a dry basis) as 61.4% C, 5.79% H2, 25.31% O2, 1.09% N2, 1.41% S, and 5% ash (non-combustibles) is burned steadily with 50% excess air in the boiler. The fuel and air enter this boiler at standard conditions and the products of combustion in the smokestack are at 127C. Assume that sulfur dioxide has an enthalpy of formation of -297.1 MJ/kmol and an average specific heat capacity at constant pressure of 41.7 kJ/kmol-K. Write a balanced chemical equation for the combustion reaction and hence determine the air fuel- ratio. Prepare a 'mass balance diagram' for the boiler. Calculate the LHV (lower heating value) combustion efficiency and the quantity of steam generated per unit mass of fuel burned.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


