Question
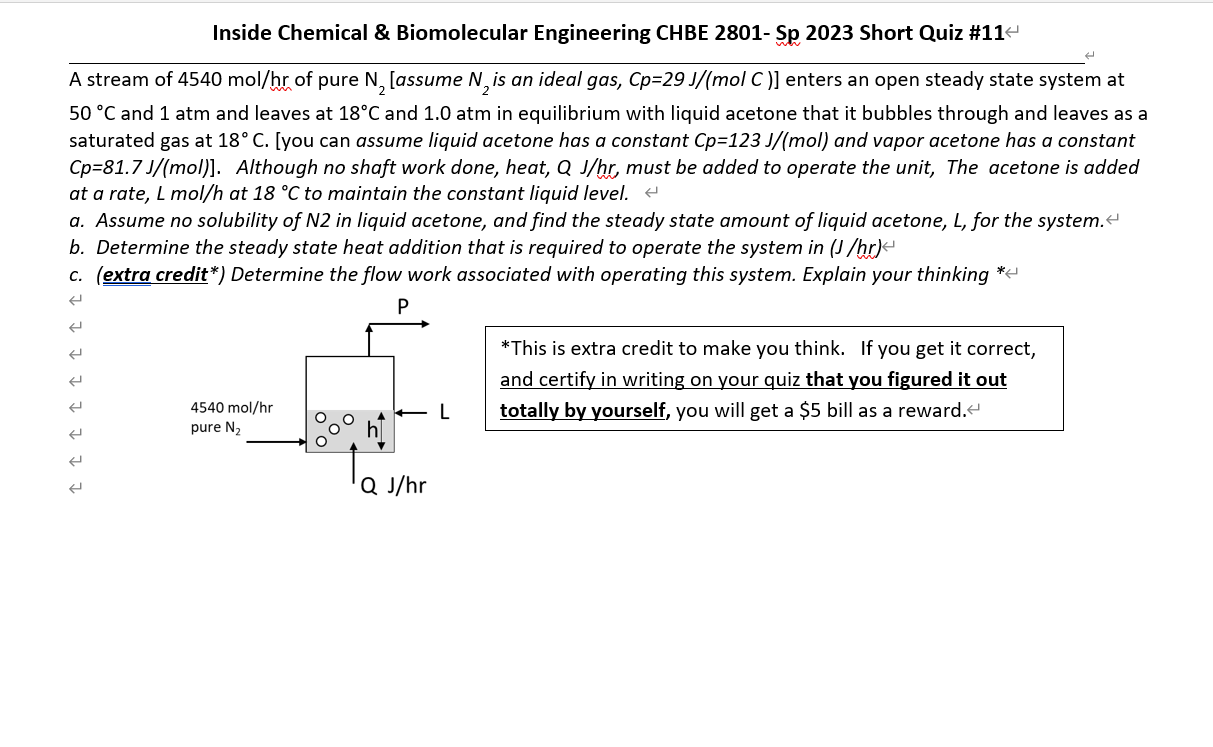
A stream of 4540 mol/hr of pure N2 [assume N2 is an ideal gas, Cp=29 J/(mol C )] enters an open steady state system at
A stream of 4540 mol/hr of pure N2 [assume N2 is an ideal gas, Cp=29 J/(mol C )] enters an open steady state system at 50 C and 1 atm and leaves at 18C and 1.0 atm in equilibrium with liquid acetone that it bubbles through and leaves as a saturated gas at 18 C. [you can assume liquid acetone has a constant Cp=123 J/(mol) and vapor acetone has a constant Cp=81.7 J/(mol)]. Although no shaft work done, heat, Q J/hr, must be added to operate the unit, The acetone is added at a rate, L mol/h at 18 C to maintain the constant liquid level. a. Assume no solubility of N2 in liquid acetone, and find the steady state amount of liquid acetone, L, for the system. b. Determine the steady state heat addition that is required to operate the system in (J /hr) c. (extra credit*) Determine the flow work associated with operating this system. Explain your thinking.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


