Question
A student conducts an experiment to determine the formula of an unknown hydrate by heating the hydrate and measuring the change in mass. She
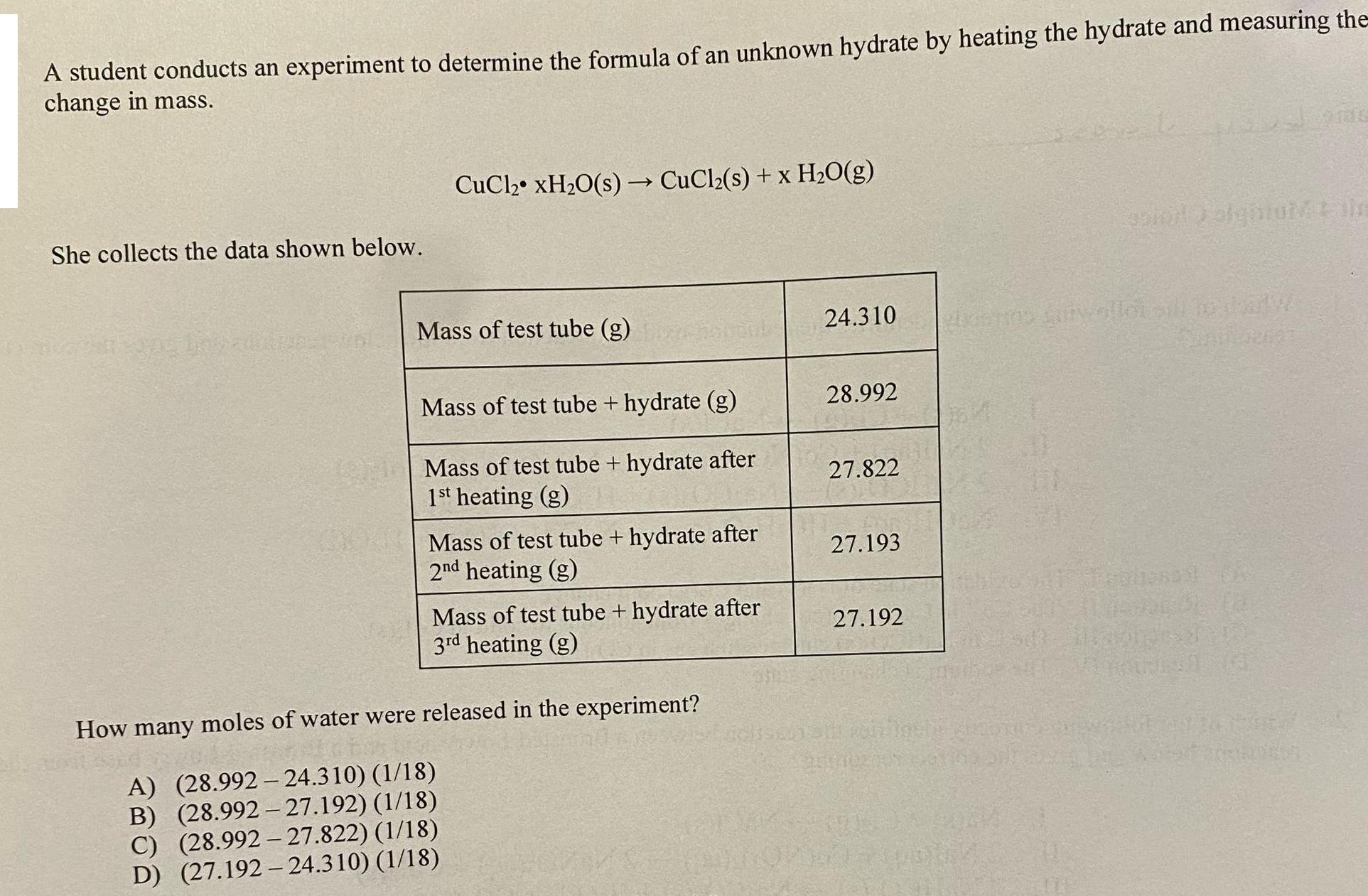
A student conducts an experiment to determine the formula of an unknown hydrate by heating the hydrate and measuring the change in mass. She collects the data shown below. CuCl, xH,O(s) CuClz(s) + xH2O(g) Mass of test tube (g) Mass of test tube + hydrate (g) Mass of test tube + hydrate after 1st heating (g) Mass of test tube + hydrate after 2nd heating (g) Mass of test tube + hydrate after 3rd heating (g) How many moles of water were released in the experiment? A) (28.992-24.310) (1/18) B) (28.992-27.192) (1/18) C) (28.992-27.822) (1/18) D) (27.192-24.310) (1/18) 24.310 28.992 27.822 27.193 27.192 Funtiores
Step by Step Solution
3.46 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below Ans The ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Biology
Authors: Mary Jones, Richard Fosbery, Jennifer Gregory, Dennis Taylor
4th Edition
1107636825, 978-1107636828
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



