Answered step by step
Verified Expert Solution
Question
1 Approved Answer
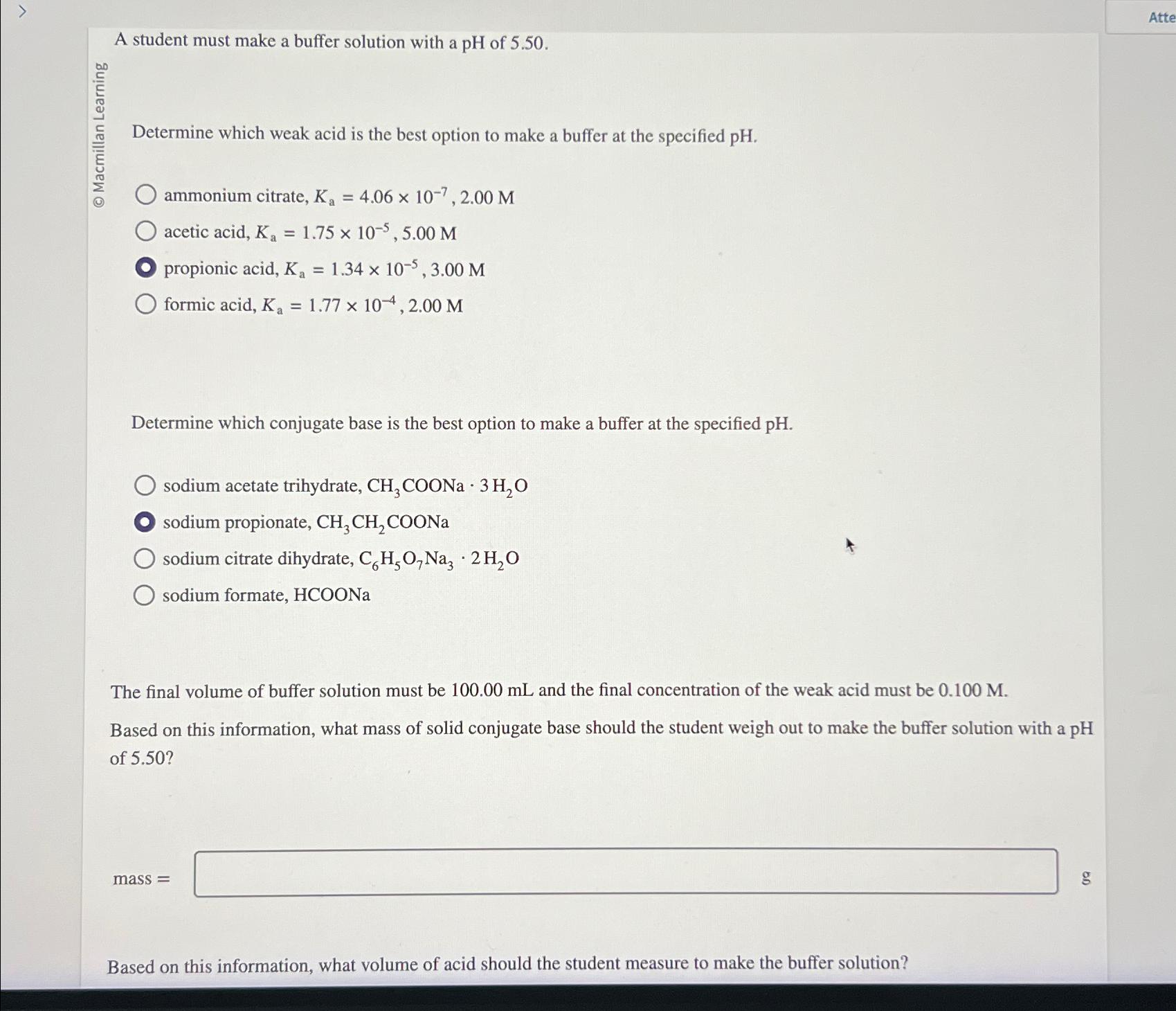
A student must make a buffer solution with a p H of 5 . 5 0 . Determine which weak acid is the best option
A student must make a buffer solution with a of
Determine which weak acid is the best option to make a buffer at the specified
ammonium citrate,
acetic acid,
propionic acid,
formic acid,
Determine which conjugate base is the best option to make a buffer at the specified pH
sodium acetate trihydrate,
sodium propionate,
sodium citrate dihydrate,
sodium formate,
The final volume of buffer solution must be and the final concentration of the weak acid must be
Based on this information, what mass of solid conjugate base should the student weigh out to make the buffer solution with a pH of
mass
g
Based on this information, what volume of acid should the student measure to make the buffer solution?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


