Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A student prepared an equilibrium system using Fe(NO3)3 and KSCN, which reacted to produce iron(III) thiocyanate by the balanced reaction shown below. Fe3+ SCN
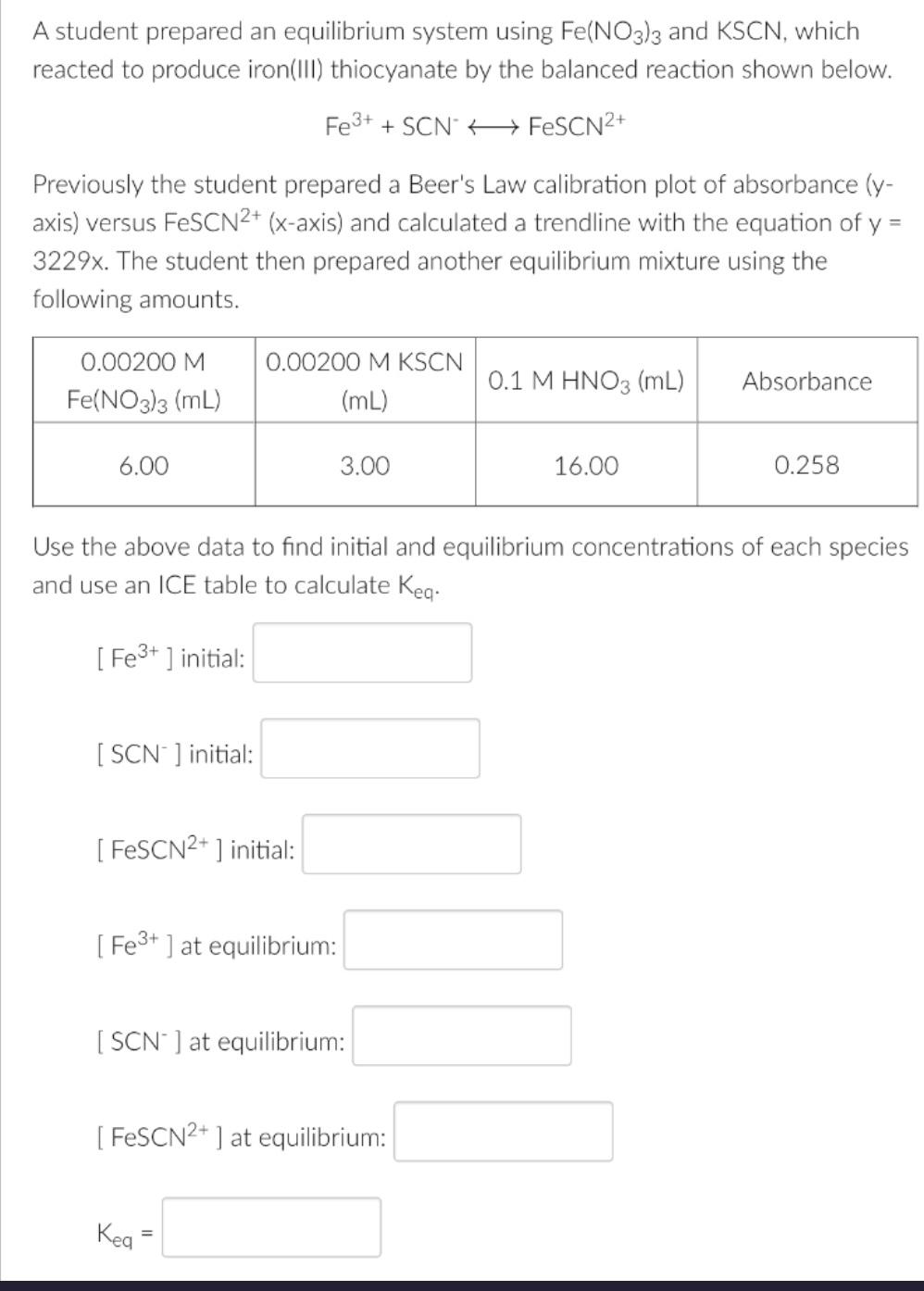
A student prepared an equilibrium system using Fe(NO3)3 and KSCN, which reacted to produce iron(III) thiocyanate by the balanced reaction shown below. Fe3+ SCN FeSCN2+ Previously the student prepared a Beer's Law calibration plot of absorbance (y- axis) versus FeSCN2+ (x-axis) and calculated a trendline with the equation of y = 3229x. The student then prepared another equilibrium mixture using the following amounts. 0.00200 M Fe(NO3)3 (mL) 0.00200 M KSCN (mL) 0.1 M HNO3 (mL) Absorbance 6.00 3.00 16.00 0.258 Use the above data to find initial and equilibrium concentrations of each species and use an ICE table to calculate Keq. [Fe3+] initial: [SCN] initial: [FeSCN+] initial: [Fe3+] at equilibrium: [SCN] at equilibrium: [FeSCN2+] at equilibrium: Keg =
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


