Question
A student titrated a 10.00 mL sample of vinegar with 0.187 M NaOH. The student analyzed their titration curve and summarized their data in
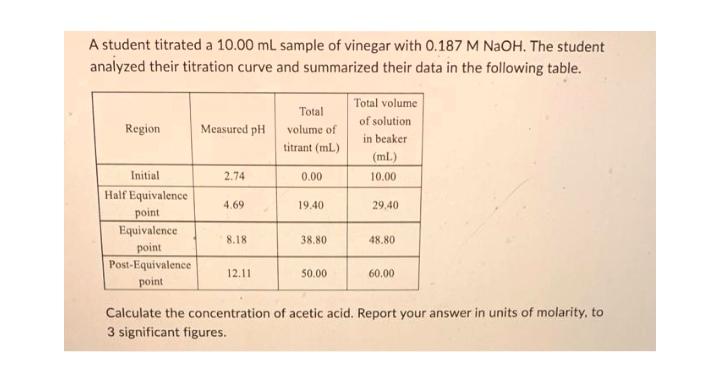
A student titrated a 10.00 mL sample of vinegar with 0.187 M NaOH. The student analyzed their titration curve and summarized their data in the following table. Region Initial Half Equivalence point Equivalence point Post-Equivalence point Measured pH 2.74 4.69 8.18 12.11 Total volume of titrant (ml) 0.00 19.40 38.80 50.00 Total volume of solution in beaker (ml) 10.00 29.40 48.80 60,00 Calculate the concentration of acetic acid. Report your answer in units of molarity, to 3 significant figures.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



