Question
(a) Suggest a structure for enolate (B) (b) Using cyclic deprotonation models, justify why the enolate (B) has a particular stereochemistry (E or Z). (8
(a) Suggest a structure for enolate (B)
(b) Using cyclic deprotonation models, justify why the enolate (B) has a particular stereochemistry (E or Z). (8 marks)
(c) Using Newman projects, account for why compound (D) has the syn stereochemical relationship between the stereogenic centres labelled 3 and 4. (8 marks)
(d) When the auxiliary is cleaved, will the product compound (E) be a single enantiomer, racemic, or achiral? Justify your answer. (4 marks)
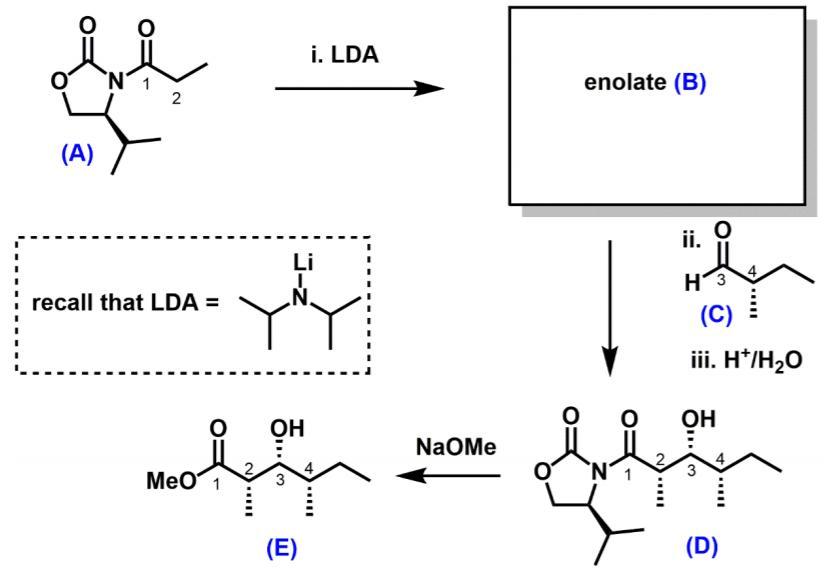
i. LDA N' enolate (B) (A) ii. Li 4 recall that LDA = H3 (C) iii. H*/H20 OH OH NaOMe MeO 1 4 3 (E) (D)
Step by Step Solution
3.55 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Marc Loudon
5th edition
981519431, 978-0981519449, 098151944X, 978-0-98151943, 978-0981519432
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



