Answered step by step
Verified Expert Solution
Question
1 Approved Answer
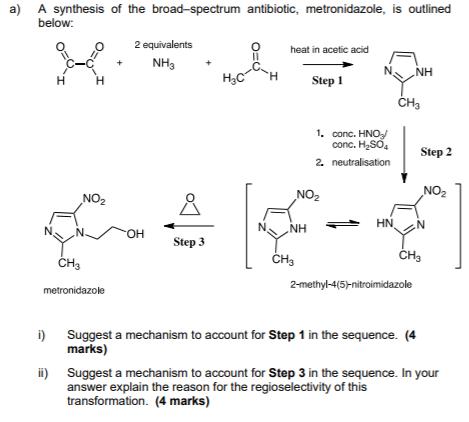
a) A synthesis of the broad-spectrum antibiotic, metronidazole, is outlined below: 2 equivalents heat in acetic acid NH3 H3C H. NH H H Step
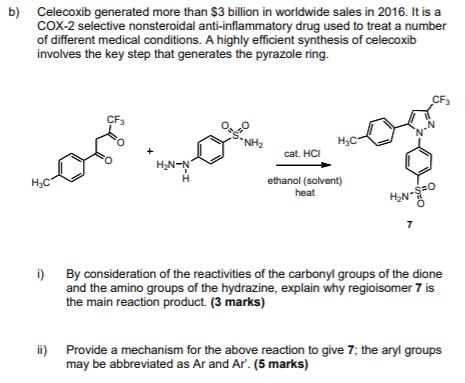
a) A synthesis of the broad-spectrum antibiotic, metronidazole, is outlined below: 2 equivalents heat in acetic acid NH3 H3C H. NH H H Step 1 1. conc. HNO conc. H,SO, Step 2 2. neutralisation NO2 NO2 NO2 HN NH HO. Step 3 CH3 2-methyl-4(5-nitroimidazole metronidazole i) Suggest a mechanism to account for Step 1 in the sequence. (4 marks) ii) Suggest a mechanism to account for Step 3 in the sequence. In your answer explain the reason for the regioselectivity of this transformation. (4 marks) b) Celecoxib generated more than $3 billion in worldwide sales in 2016. It is a CoX-2 selective nonsteroidal anti-inflammatory drug used to treat a number of different medical conditions. A highly efficient synthesis of celecoxib involves the key step that generates the pyrazole ring. CF3 CF3 NH2 H3C cat. HCI H2N-N H,C H ethanol (solvent) heat 7 i) By consideration of the reactivities of the carbonyl groups of the dione and the amino groups of the hydrazine, explain why regioisomer 7 is the main reaction product. (3 marks) ii) Provide a mechanism for the above reaction to give 7; the aryl groups may be abbreviated as Ar and Ar. (5 marks)
Step by Step Solution
★★★★★
3.52 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
a i The above step 1 illustrates the reaction between 1 mol of glyoxal 2 mols of ammonia and 1 mol o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started