Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) () () + () table: 298K what is at 100C does temp increase make reaction more spontanous or not b) +() + +() +()
a) () () + ()
table: 298K
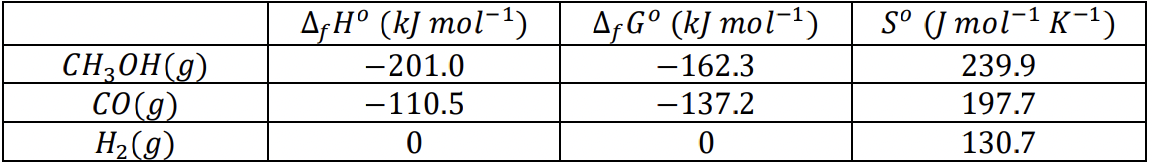 what is at 100C
what is at 100C
does temp increase make reaction more spontanous or not
b) +() + +() +() + +()
table: 298K
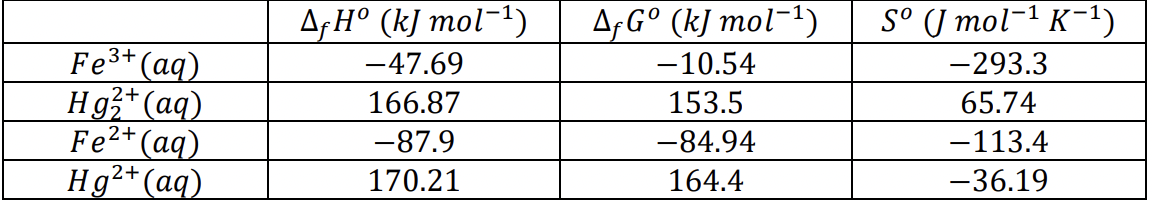 what is equilibrium constant K at 25C
what is equilibrium constant K at 25C
what is G at 25C when:
[2 2+] = 0.185 1 , [ 3+] = 0.015 1
[ 2+] = 0.010 1 , [ 2+] = 0.030 1
if increase in Fe2+ concentration, would it make the reaction spontanous or not and why
CH2OH(g) CO(g) H2(g) AfH (kJ mol-1) -201.0 -110.5 0 AfG (kJ mol-1) -162.3 - 137.2 0 S ( mol-1 K-1) 239.9 197.7 130.7 - Fe3+ (aq) Hg2+ (aq) Fe2+ (aq) Hg2+ (aq) A H (kJ mol-1) -47.69 166.87 -87.9 170.21 A,G (kJ mol-1) -10.54 153.5 -84.94 164.4 S ( mol-1 K-1) -293.3 65.74 -113.4 -36.19Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


