Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a. Take B C, then convert it to Fahrenheit and Kelvin. b. Find the amount of heat needed to change the temperature of B
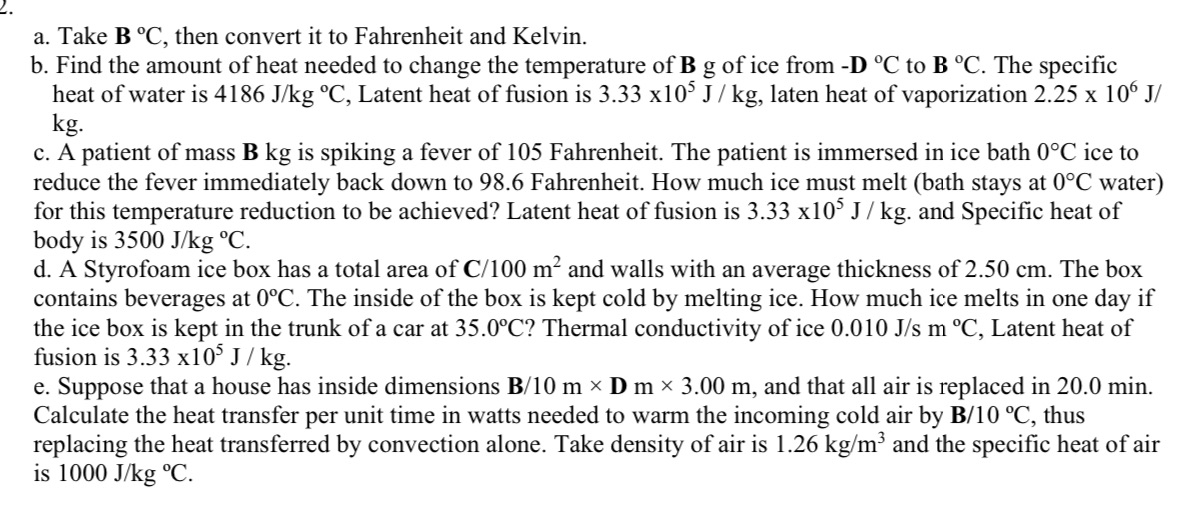
a. Take B C, then convert it to Fahrenheit and Kelvin. b. Find the amount of heat needed to change the temperature of B g of ice from -D C to B C. The specific heat of water is 4186 J/kg C, Latent heat of fusion is 3.33 x105 J / kg, laten heat of vaporization 2.25 x 106 J/ kg. c. A patient of mass B kg is spiking a fever of 105 Fahrenheit. The patient is immersed in ice bath 0C ice to reduce the fever immediately back down to 98.6 Fahrenheit. How much ice must melt (bath stays at 0C water) for this temperature reduction to be achieved? Latent heat of fusion is 3.33 x105 J/kg. and Specific heat of body is 3500 J/kg C. d. A Styrofoam ice box has a total area of C/100 m and walls with an average thickness of 2.50 cm. The box contains beverages at 0C. The inside of the box is kept cold by melting ice. How much ice melts in one day if the ice box is kept in the trunk of a car at 35.0C? Thermal conductivity of ice 0.010 J/s m C, Latent heat of fusion is 3.33 x105 J/kg. e. Suppose that a house has inside dimensions B/10 m D m 3.00 m, and that all air is replaced in 20.0 min. Calculate the heat transfer per unit time in watts needed to warm the incoming cold air by B/10 C, thus replacing the heat transferred by convection alone. Take density of air is 1.26 kg/m and the specific heat of air is 1000 J/kg C.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


