Answered step by step
Verified Expert Solution
Question
1 Approved Answer
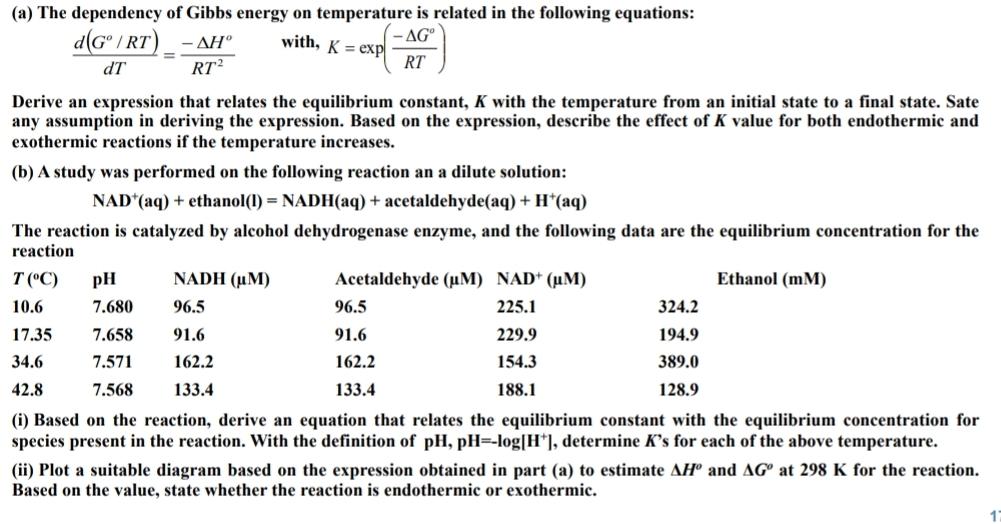
( a ) The dependency of Gibbs energy on temperature is related in the following equations: d ( G o R T ) d T
a The dependency of Gibbs energy on temperature is related in the following equations:
with, exp
Derive an expression that relates the equilibrium constant, with the temperature from an initial state to a final state. Sate any assumption in deriving the expression. Based on the expression, describe the effect of value for both endothermic and exothermic reactions if the temperature increases.
b A study was performed on the following reaction an a dilute solution:
ethanolNADH
The reaction is catalyzed by alcohol dehydrogenase enzyme, and the following data are the equilibrium concentration for the reaction
tableNADHAcetaldehyde Ethanol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


