Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) The Raman spectrum of the heterogeneous catalyst, titanium silicalite-1, displays two vibrational bands at 380 cm*1 and 800 cm-1. However, when reacted with
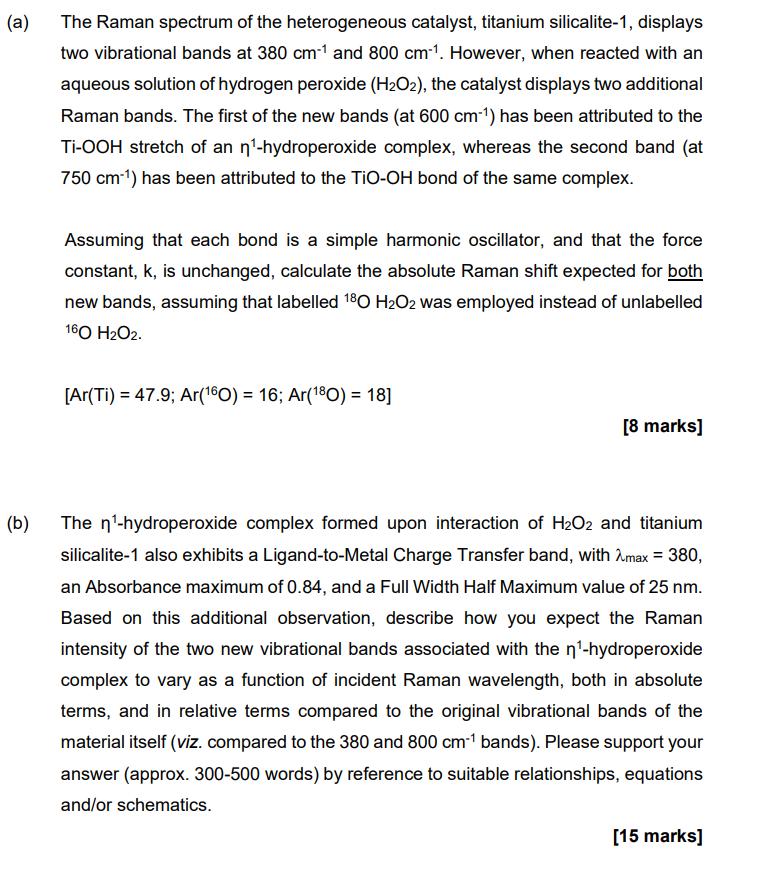
(a) The Raman spectrum of the heterogeneous catalyst, titanium silicalite-1, displays two vibrational bands at 380 cm*1 and 800 cm-1. However, when reacted with an aqueous solution of hydrogen peroxide (H2O2), the catalyst displays two additional Raman bands. The first of the new bands (at 600 cm-1) has been attributed to the Ti-OOH stretch of an n-hydroperoxide complex, whereas the second band (at 750 cm-1) has been attributed to the TiO-OH bond of the same complex. Assuming that each bond is a simple harmonic oscillator, and that the force constant, k, is unchanged, calculate the absolute Raman shift expected for both new bands, assuming that labelled 180 H2O2 was employed instead of unlabelled 160 H2O2. [Ar(Ti) 47.9; Ar(160) = 16; Ar(180) = 18] [8 marks] (b) The n-hydroperoxide complex formed upon interaction of H2O2 and titanium silicalite-1 also exhibits a Ligand-to-Metal Charge Transfer band, with max = 380, an Absorbance maximum of 0.84, and a Full Width Half Maximum value of 25 nm. Based on this additional observation, describe how you expect the Raman intensity of the two new vibrational bands associated with the n-hydroperoxide complex to vary as a function of incident Raman wavelength, both in absolute terms, and in relative terms compared to the original vibrational bands of the material itself (viz. compared to the 380 and 800 cm-1 bands). Please support your answer (approx. 300-500 words) by reference to suitable relationships, equations and/or schematics. [15 marks]
Step by Step Solution
★★★★★
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
a To calculate the absolute Raman shift expected for both new bands we can use the formula 1 2c x k ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


