Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A thermodynamic cycle consists of three processes. The physical cycle is a piston/cylinder system containing air at the starting condition of T=300 K, P=100
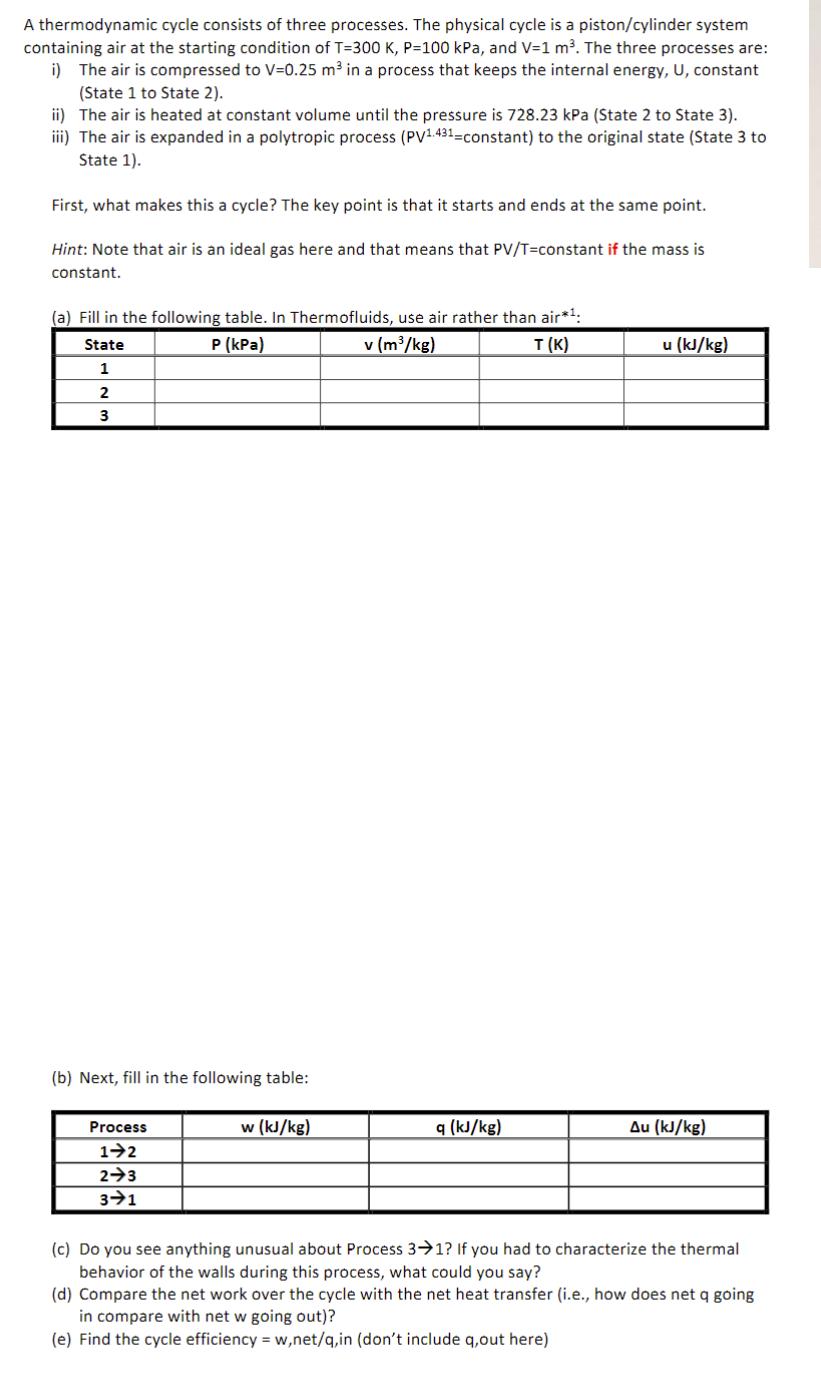
A thermodynamic cycle consists of three processes. The physical cycle is a piston/cylinder system containing air at the starting condition of T=300 K, P=100 kPa, and V=1 m. The three processes are: i) The air is compressed to V=0.25 m in a process that keeps the internal energy, U, constant (State 1 to State 2). ii) The air is heated at constant volume until the pressure is 728.23 kPa (State 2 to State 3). iii) The air is expanded in a polytropic process (PV1.431-constant) to the original state (State 3 to State 1). First, what makes this a cycle? The key point is that it starts and ends at the same point. Hint: Note that air is an ideal gas here and that means that PV/T=constant if the mass is constant. (a) Fill in the following table. In Thermofluids, use air rather than air*: P (kPa) v (m/kg) T(K) State 1 2 3 (b) Next, fill in the following table: Process 1-2 2-3 3-1 w (kJ/kg) q (kJ/kg) u (kJ/kg) Au (kJ/kg) (c) Do you see anything unusual about Process 3-1? If you had to characterize the thermal behavior of the walls during this process, what could you say? (d) Compare the net work over the cycle with the net heat transfer (i.e., how does net q going in compare with net w going out)? (e) Find the cycle efficiency = w,net/q,in (don't include q,out here)
Step by Step Solution
★★★★★
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


