Question
A three-stage batch rectifier (first stage is the still pot) is charged with 100kmol of a 20 mol% n-hexane in n-octane mixture. At a

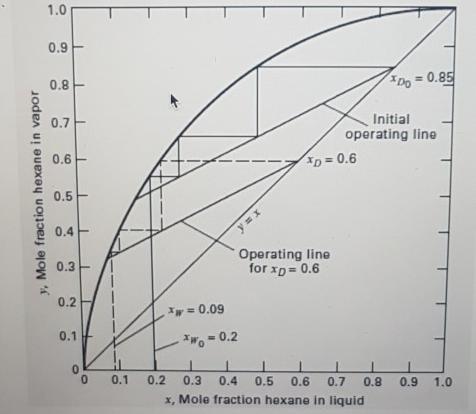
A three-stage batch rectifier (first stage is the still pot) is charged with 100kmol of a 20 mol% n-hexane in n-octane mixture. At a constant-reflux ratio of 1(L/V=0.5), how many moles of charge must be distilled for an average product composition of 70 mol% nC6? The phase equilibrium curve at column pressure is given in Figure below. If the boilup rate is 10 kmol/h, calculate distillation time. 1.0 0.9 0.8 Do = 0.85 0.7 Initial operating line 0.6 D = 0.6 0.5 0.4 Operating line for xp= 0.6 0.3- 0.2 = 0.09 0.1 = 0.2 0.1 0.2 0.3 0.4 0,5 0.6 0.7 0.8 0.9 1.0 x, Mole fraction hexane in liquid y, Mole fraction hexane in vapor
Step by Step Solution
3.39 Rating (143 Votes )
There are 3 Steps involved in it
Step: 1
From the given diagram xW0 020 xW 009 Develop a table for the composition in distillate and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Physics
Authors: Jearl Walker, Halliday Resnick
9th Edition
470469080, 470556536, 470469110, 9780470469088, 9780470556535, 978-0470469118
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



