Question
A tin chloride Q undergoes the following reactions (not balanced) Q+CIX Q+ MeN Y Q+CuCl Z+CuCl X is a monoanion having pyramidal geometry. Both
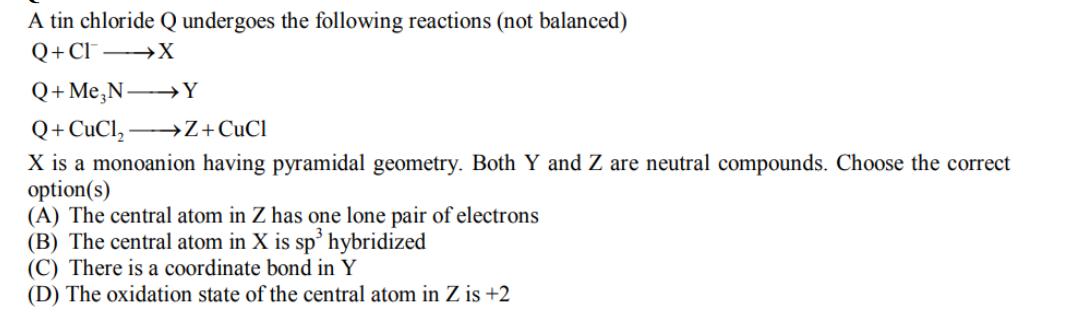
A tin chloride Q undergoes the following reactions (not balanced) Q+CIX Q+ MeN Y Q+CuCl Z+CuCl X is a monoanion having pyramidal geometry. Both Y and Z are neutral compounds. Choose the correct option(s) (A) The central atom in Z has one lone pair of electrons (B) The central atom in X is sp hybridized (C) There is a coordinate bond in Y (D) The oxidation state of the central atom in Z is +2
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Paula Yurkanis Bruice
4th edition
131407481, 978-0131407480
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



