Answered step by step
Verified Expert Solution
Question
1 Approved Answer
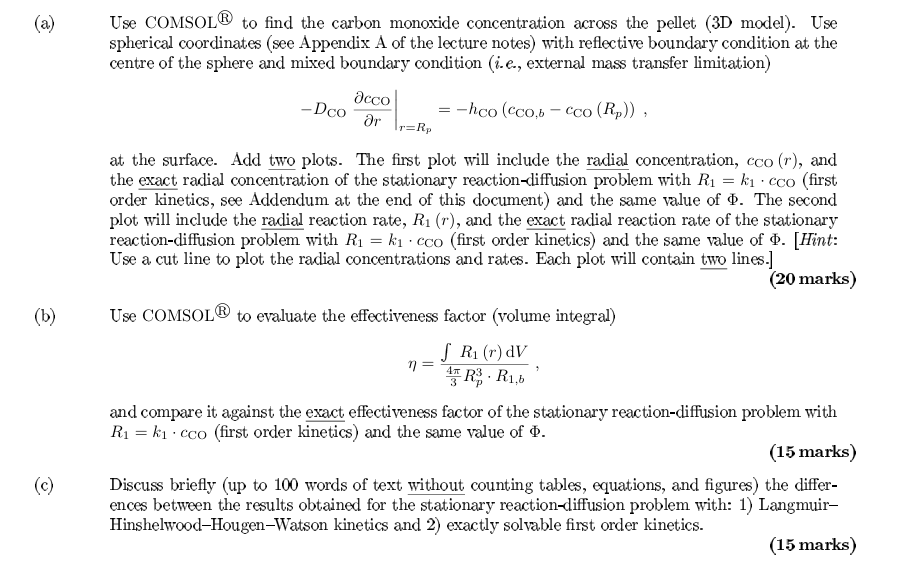
( a ) Use COMSOL ? to find the carbon monoxide concentration across the pellet ( 3 D model ) . Use spherical coordinates (
a Use COMSOL to find the carbon monoxide concentration across the pellet D model Use
spherical coordinates see Appendix A of the lecture notes with reflective boundary condition at the
centre of the sphere and mixed boundary condition ie external mass transfer limitation
at the surface. Add two plots. The first plot will include the radial concentration, and
the exact radial concentration of the stationary reactiondiffusion problem with first
order kinetics, see Addendum at the end of this document and the same value of The second
plot will include the radial reaction rate, and the exact radial reaction rate of the stationary
reactiondiffusion problem with first order kinetics and the same value of Hint:
Use a cut line to plot the radial concentrations and rates. Each plot will contain two lines.
marks
b Use COMSOL to evaluate the effectiveness factor volume integral
and compare it against the exact effectiveness factor of the stationary reactiondiffusion problem with
first order kinetics and the same value of
marks
c Discuss briefly up to words of text without counting tables, equations, and figures the differ
ences between the results obtained for the stationary reactiondiffusion problem with: Langmuir
HinshelwoodHougenWatson kinetics and exactly solvable first order kinetics.
marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


