Answered step by step
Verified Expert Solution
Question
1 Approved Answer
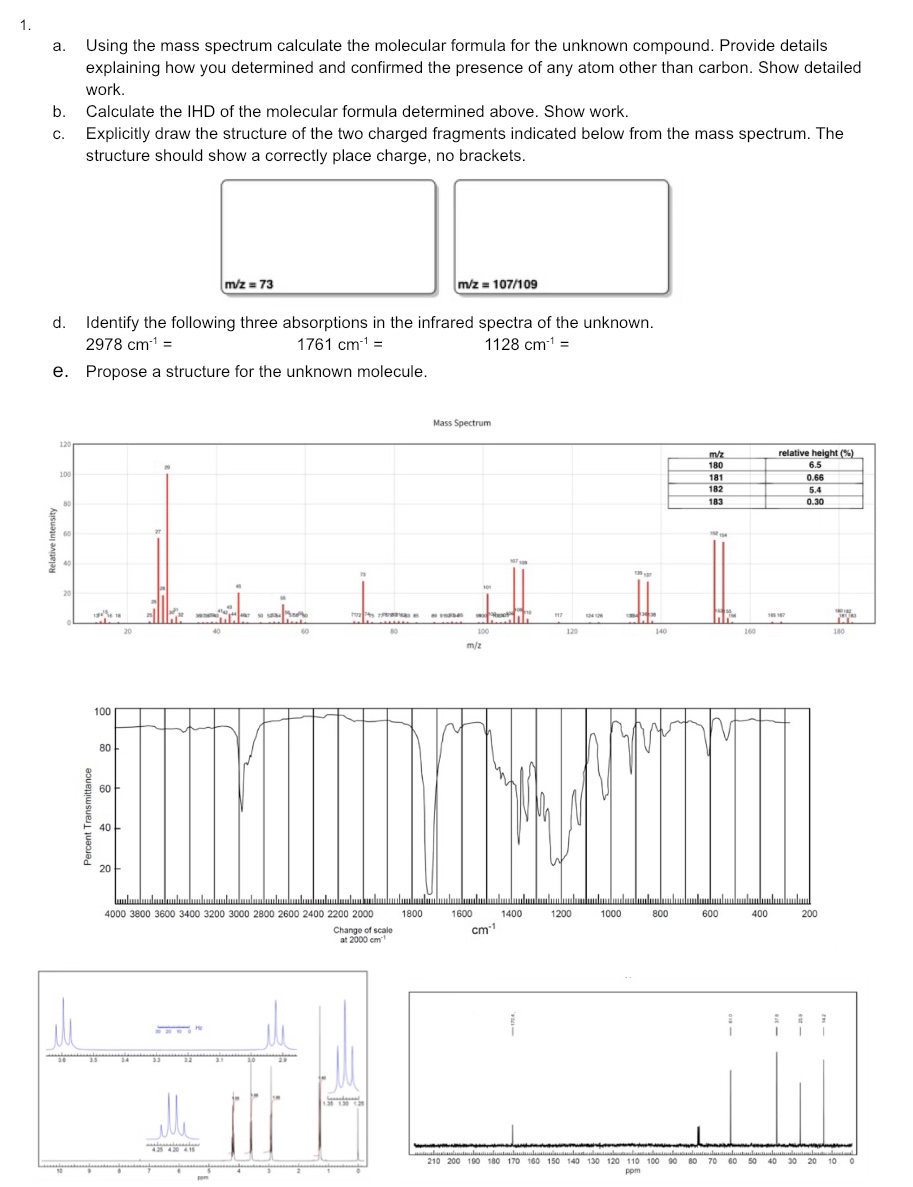
a . Using the mass spectrum calculate the molecular formula for the unknown compound. Provide details explaining how you determined and confirmed the presence of
a Using the mass spectrum calculate the molecular formula for the unknown compound. Provide details explaining how you determined and confirmed the presence of any atom other than carbon. Show detailed work.
b Calculate the IHD of the molecular formula determined above. Show work.
c Explicitly draw the structure of the two charged fragments indicated below from the mass spectrum. The structure should show a correctly place charge, no brackets.
d Identify the following three absorptions in the infrared spectra of the unknown.
e Propose a structure for the unknown molecule.
Mass Spectrum
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


