Question
a) Using your knowledge of organic reaction mechanisms, explain the formation of EACH product (A,B) in the following reaction. This will require you to
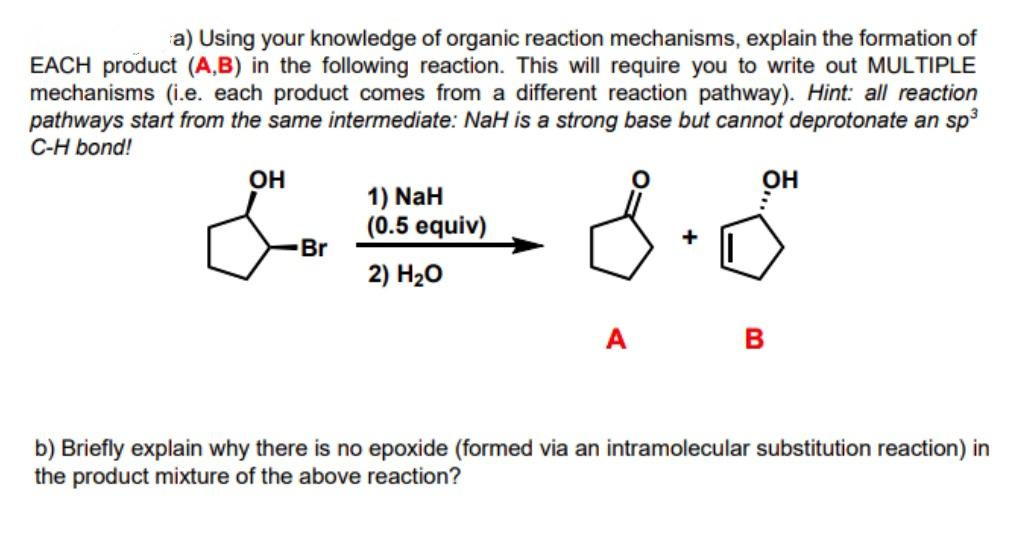
a) Using your knowledge of organic reaction mechanisms, explain the formation of EACH product (A,B) in the following reaction. This will require you to write out MULTIPLE mechanisms (i.e. each product comes from a different reaction pathway). Hint: all reaction pathways start from the same intermediate: NaH is a strong base but cannot deprotonate an sp3 C-H bond! OH OH 1) NaH (0.5 equiv) Br + 2) H20 A B b) Briefly explain why there is no epoxide (formed via an intramolecular substitution reaction) in the product mixture of the above reaction?
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Accounting for Decision Making and Control
Authors: Jerold Zimmerman
8th edition
78025745, 978-0078025747
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



