Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A water tank has one rigid wall and one movable wall that acts like a massless piston. The tank contains a volume of water
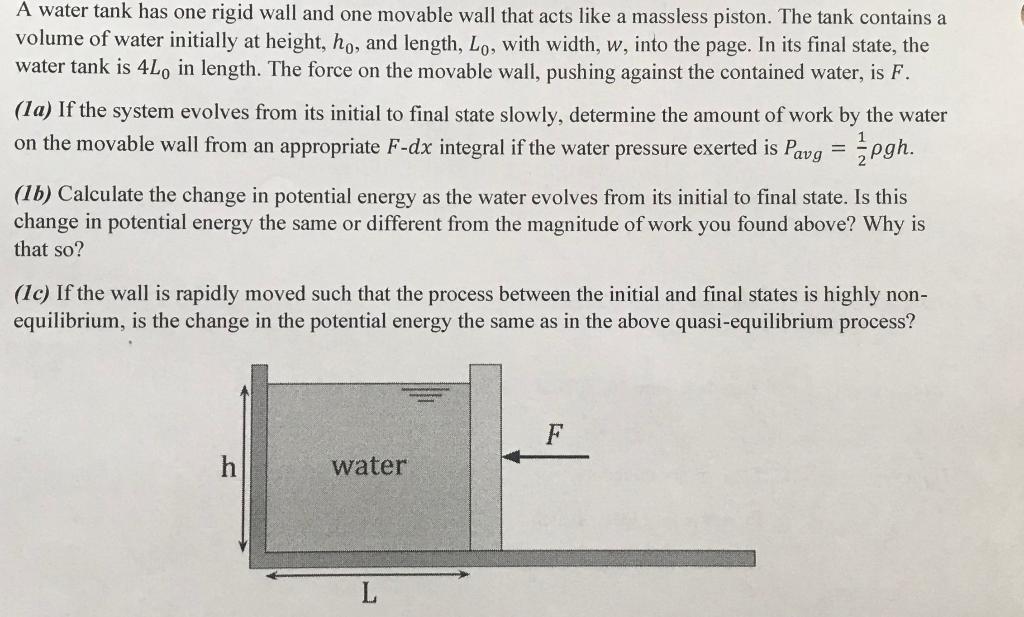
A water tank has one rigid wall and one movable wall that acts like a massless piston. The tank contains a volume of water initially at height, ho, and length, Lo, with width, w, into the page. In its final state, the water tank is 4Lo in length. The force on the movable wall, pushing against the contained water, is F. (1a) If the system evolves from its initial to final state slowly, determine the amount of work by the water on the movable wall from an appropriate F-dx integral if the water pressure exerted is Pavg = pgh. (16) Calculate the change in potential energy as the water evolves from its initial to final state. Is this change in potential energy the same or different from the magnitude of work you found above? Why is that so? (1c) If the wall is rapidly moved such that the process between the initial and final states is highly non- equilibrium, is the change in the potential energy the same as in the above quasi-equilibrium process? h water
Step by Step Solution
★★★★★
3.38 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
1a Work done by the water on the movable wall is given by W F dx Pavg w dx Since force F Pressure x ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


