Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A well - insulated container consists of two halves of equal volumes V separated by a partition. On one half, there are N moles of
A wellinsulated container consists of two halves of equal volumes V separated by a partition. On one half, there are N moles of a monatomic ideal gas at initial temperature Ti On the other, there is a vacuum. The partition is suddenly removed, and the gas expands. Recall for a monatomic ideal gas that PV N kB T and E NkB T
Expected answer is shown below, please show your work.
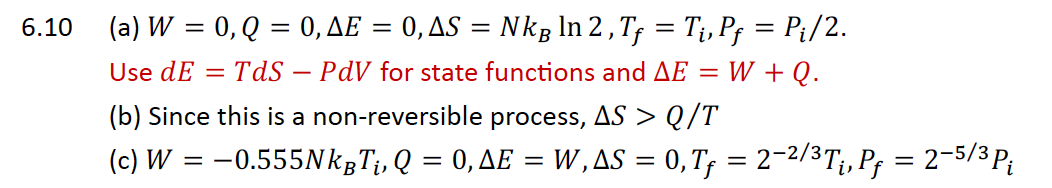
a
Use for state functions and
b Since this is a nonreversible process,
c
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


