A. What is the relationship between volume and pressure if temperature of gas is held constant? B. What is relationship between volume and temperature if
A. What is the relationship between volume and pressure if temperature of gas is held constant?
B. What is relationship between volume and temperature if pressure of gas is held constant?
C. What is relationship between volume and temperature if the pressure and temperature of gas is held constant?
Based on the data, write equation to describe relationship between two states of P, V, and T if the number of moles are held constant.
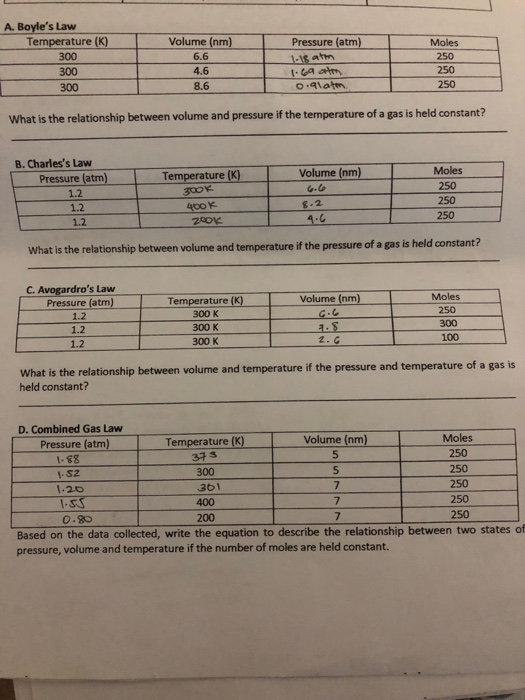
A. Boyle's Law Temperature (K) 300 300 300 Pressure (atm) 1-18 atm 1.69 atm 0.91@tm. What is the relationship between volume and pressure if the temperature of a gas is held constant? B. Charles's Law Pressure (atm) 1.2 1.2 1.2 Volume (nm) 6.6 4.6 8.6 C. Avogardro's Law Pressure (atm) 1.2 1.2 1.2 Temperature (K) 300k 400 k 8.2 4-6 What is the relationship between volume and temperature if the pressure of a gas is held constant? 200K Temperature (K) 300 K 300 K 300 K Volume (nm) Volume (nm) G.6 4.8 2. G Temperature (K) 373 300 361 400 Moles 250 250 250 What is the relationship between volume and temperature if the pressure and temperature of a gas is held constant? Volume (nm) 5 Moles 250 250 250 D. Combined Gas Law Pressure (atm) 1-88 1.52 1.20 1.55 0.80 200 7 250 Based on the data collected, write the equation to describe the relationship between two states of pressure, volume and temperature if the number of moles are held constant. 5 7 7 Moles 250 300 100 Moles 250 250 250 250
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


