Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) What reaction schemes and conditions would you use to maximize the selectivity parameters S for the following reactions: A+CDA+CUrD=15exp(273/T)CA0.5CCrU=200exp(2000/T)CACC where D is the desired
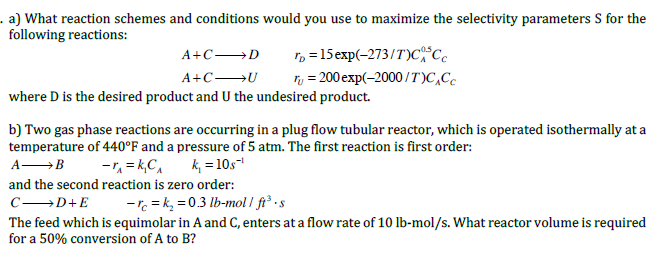 a) What reaction schemes and conditions would you use to maximize the selectivity parameters S for the following reactions: A+CDA+CUrD=15exp(273/T)CA0.5CCrU=200exp(2000/T)CACC where D is the desired product and U the undesired product. b) Two gas phase reactions are occurring in a plug flow tubular reactor, which is operated isothermally at a temperature of 440F and a pressure of 5atm. The first reaction is first order: ABrA=k1CAk1=10s1 and the second reaction is zero order: CD+ErC=k2=0.3lbmol/ft3s The feed which is equimolar in A and C, enters at a flow rate of 10lbmol/s. What reactor volume is required for a 50% conversion of A to B
a) What reaction schemes and conditions would you use to maximize the selectivity parameters S for the following reactions: A+CDA+CUrD=15exp(273/T)CA0.5CCrU=200exp(2000/T)CACC where D is the desired product and U the undesired product. b) Two gas phase reactions are occurring in a plug flow tubular reactor, which is operated isothermally at a temperature of 440F and a pressure of 5atm. The first reaction is first order: ABrA=k1CAk1=10s1 and the second reaction is zero order: CD+ErC=k2=0.3lbmol/ft3s The feed which is equimolar in A and C, enters at a flow rate of 10lbmol/s. What reactor volume is required for a 50% conversion of A to B Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


