Question
a) With the aid of equations briefly outline why cyclopentadiene is acidic and will readily lose one of the hydrogens shown; but pyrrole is
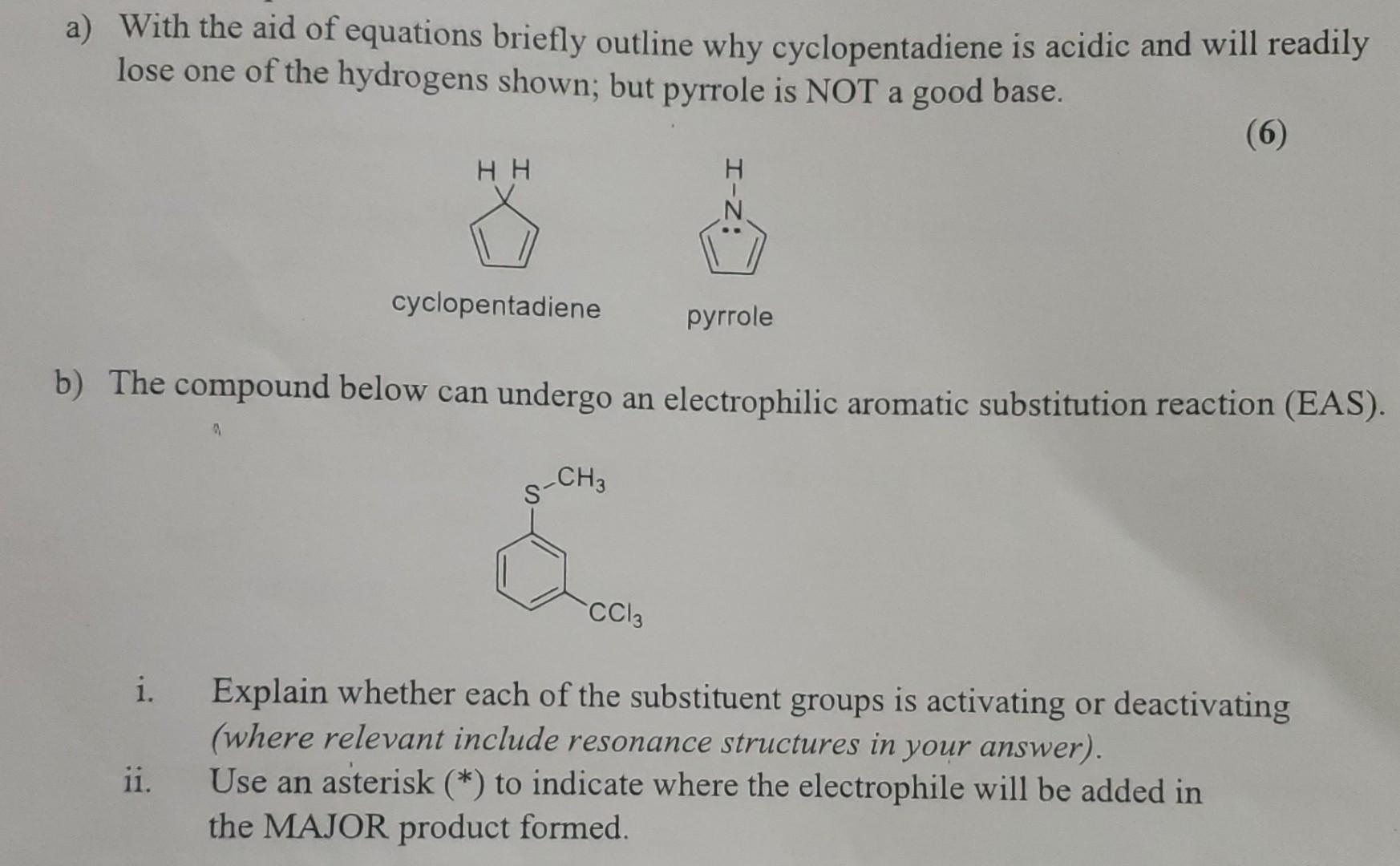
a) With the aid of equations briefly outline why cyclopentadiene is acidic and will readily lose one of the hydrogens shown; but pyrrole is NOT a good base. (6) i. ii. HH b) The compound below can undergo an electrophilic aromatic substitution reaction (EAS). a cyclopentadiene CH3 pyrrole CC13 Explain whether each of the substituent groups is activating or deactivating (where relevant include resonance structures in your answer). Use an asterisk (*) to indicate where the electrophile will be added in the MAJOR product formed.
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
Based on the provided questions lets tackle them one at a time a Cyclopentadiene is acidic due to the ability of the resulting anion to be stabilized ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



