Question
(a) Write a mathematical expression that can be used to determine the value of S, the molar solubility of Pbl2(s). (b) If Pbl2(s) is
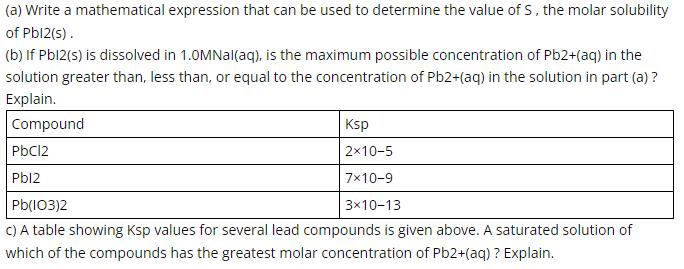
(a) Write a mathematical expression that can be used to determine the value of S, the molar solubility of Pbl2(s). (b) If Pbl2(s) is dissolved in 1.0MNal(aq), is the maximum possible concentration of Pb2+(aq) in the solution greater than, less than, or equal to the concentration of Pb2+(aq) in the solution in part (a) ? Explain. Compound Ksp PbC12 2x10-5 Pbl2 7x10-9 3x10-13 C) A table showing Ksp values for several lead compounds is given above. A saturated solution of Pb(I03)2 which of the compounds has the greatest molar concentration of Pb2+(aq) ? Explain.
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Answer The mixing of the twocomponent is called solutions The two ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Materials and process in manufacturing
Authors: E. Paul DeGarmo, J T. Black, Ronald A. Kohser
9th edition
471656534, 978-0471033066, 471033065, 978-0471656531
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



