Question
A1.The molar volume (cmmol) of a binary liquid mixture at T and P is given by: V=120X +70X+(15X +8X2)X a. Find expressions for the
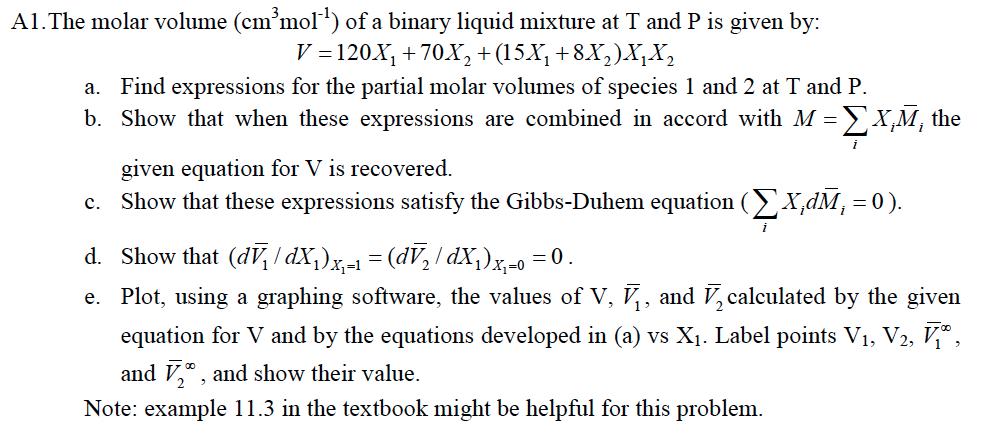
A1.The molar volume (cmmol) of a binary liquid mixture at T and P is given by: V=120X +70X+(15X +8X2)X a. Find expressions for the partial molar volumes of species 1 and 2 at T and P. b. Show that when these expressions are combined in accord with M = X,M, the c. Show that these expressions satisfy the Gibbs-Duhem equation (X,dM = 0). given equation for V is recovered. d. Show that (dv/dX)x- = (d / dX)x-0 = 0. e. Plot, using a graphing software, the values of V, V, and V, calculated by the given equation for V and by the equations developed in (a) vs X. Label points V1, V2, , and 2, and show their value. Note: example 11.3 in the textbook might be helpful for this problem.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Chemical Engineering Thermodynamics
Authors: J. M. Smith, H. C. Van Ness, M. M. Abbott
7th edition
71247084, 978-0071247085
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



