Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Above is an example of the catalytic dehydration of methanol to form 30,000 tons of Dimethyl ether. Provide the same for the production of 20,000
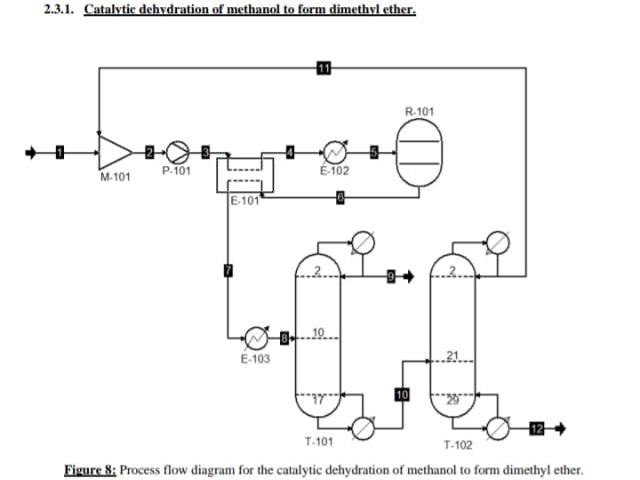
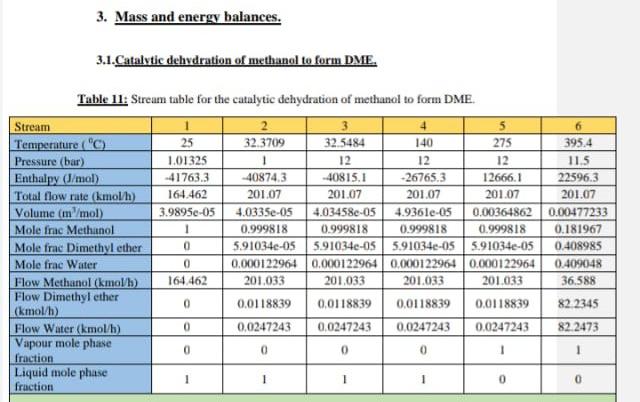
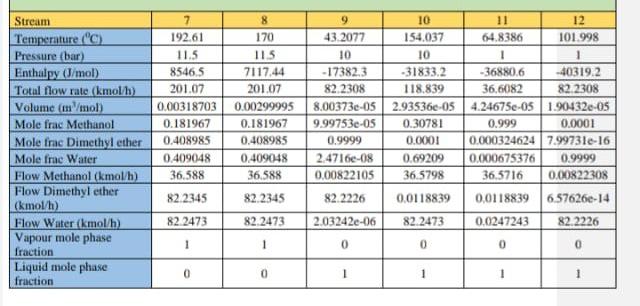
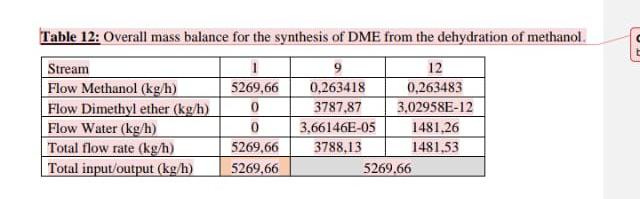
Above is an example of the catalytic dehydration of methanol to form 30,000 tons of Dimethyl ether.
Provide the same for the production of 20,000 tons of Acrylic Acid from the oxidation of Propylene
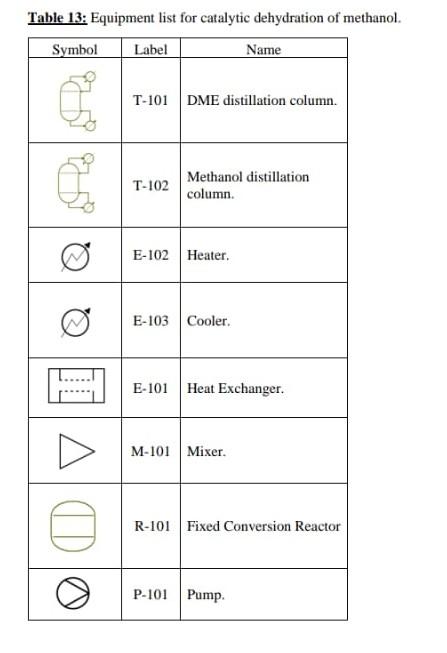
2.3.1. Catalytic dehydration of methanol to form dimethyl ether. R.101 M-101 P-101 6-102 E. 101 6.103 .21. 12 T.101 T-102 Figure 8: Process flow diagram for the catalytic dehydration of methanol to form dimethyl ether. 3. Mass and energy balances. 3.1.Catalytic dehydration of methanol to form DME. Table 11: Stream table for the catalytic dehydration of methanol to form DME. Stream 6 Temperature (C) 25 32.3709 32.5484 140 275 395.4 Pressure (bar) 1.01325 1 12 12 12 11.5 Enthalpy (l/mol) -41763.3 -40874.3 -40815.1 26765.3 12666.1 22596.3 Total flow rate (kmol/h) 164.462 201.07 201.07 201.07 201.07 201.07 Volume (in/mol) 3.9895e-05 4.0335e-05 4.03458e-05 4.936le-05 0.00364862 0.00477233 Mole frac Methanol 1 0.999818 0.999818 0.999818 0.999818 0.181967 Mole frac Dimethyl ether 0 5.91034e-05 5910340-05 5.91034e-055.91034e-05 0.408985 Mole frac Water 0 0.000122964 0.000122964 0,000122964 0.000122964 0.409048 Flow Methanol (kmol/l) 164.462 201.033 201.033 201.033 201.033 36,588 Flow Dimethyl ether 0.0118839 (kmol/h) 0.0118839 0.0118839 0.0118839 82.2345 Flow Water (kmol/b) 0 0.0247243 0.0247243 0,0247243 0.0247243 82 2473 Vapour mole phase 0 0 0 0 fraction Liquid mole phase 1 1 1 0 0 fraction Stream Temperature (C) Pressure (bar) Enthalpy (J/mol Total flow rate (kmol/h) Volume (in /mol) Mole frac Methanol Mole frac Dimethyl ether Mole frac Water Flow Methanol (kmol/h) Flow Dimethyl ether (kmol/h) Flow Water (kmol/h) Vapour mole phase fraction Liquid mole phase fruction 7 192.61 11.5 8546,5 201.07 0.00318703 0.181967 0.408985 0.409048 36,588 8 170 115 7117.44 201.07 0.00299995 0.181967 0.408985 0.409048 36,588 9 10 11 12 43.2077 154.037 64.8386 101.998 10 10 1 - 17382.3 -31833.2 -36880.6 -40319.2 82.2308 118.839 36,6082 82.2308 8.00373e-05 2.93536e-054.24675e-05 1.90432e-05 9.99753-05 0.30781 0.999 0.0001 0.9999 0.0001 0.000324624 799731e-16 2.4716-08 0.69209 0.000675376 0.9999 0.00822105 36,5798 36,5716 0.00822308 82.2226 0.0118839 0,0118839 6.57626-14 2.03242e-06 82.2473 0.0247243 82.2226 82.2345 82.2345 82.2473 82.2473 1 1 0 0 0 0 0 0 1 1 1 Table 12: Overall mass balance for the synthesis of DME from the dehydration of methanol, Stream Flow Methanol (kg/h) Flow Dimethyl ether (kg/h) Flow Water (kg/h) Total flow rate (kg/h) Total input/output (kg/h) 5269,66 0 0 5269,66 5269.66 12 0,263418 0,263483 3787,87 3,02958E-12 3,66146E-05 1481,26 3788,13 1481,53 5269.66 Table 13: Equipment list for catalytic dehydration of methanol. Symbol Label Name T-101 DME distillation column. T-102 Methanol distillation column. E-102 Heater. E-103 Cooler B E-101 Heat Exchanger. M-101 Mixer. A R-101 Fixed Conversion Reactor DO P-101 PumpStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


