Question
According to the ideal gas law, a 0.9249 mol sample of xenon gas in a 1.135 L container at 269.1 K should exert a
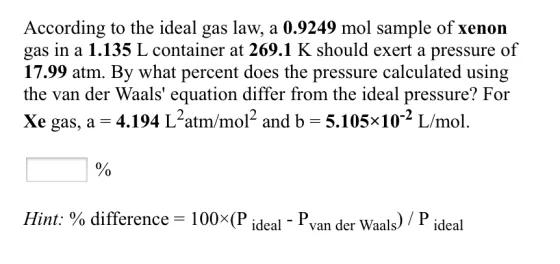
According to the ideal gas law, a 0.9249 mol sample of xenon gas in a 1.135 L container at 269.1 K should exert a pressure of 17.99 atm. By what percent does the pressure calculated using the van der Waals' equation differ from the ideal pressure? For Xe gas, a = 4.194 L?atm/mol? and b = 5.105x10-2 L/mol. % Hint: % difference = 100(P ideal - Pvan der Waals) / P ideal
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Van der Waals equation an P V2 V nb nRT substituting the values we get 4194...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Physics
Authors: Jerry D. Wilson, Anthony J. Buffa, Bo Lou
7th edition
9780321571113, 321601831, 978-0321601834
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



