An Otto cycle engine has a compression ratio of 9 and uses CH18 octane as fuel. Since the lowest calorific value of LHV fuel
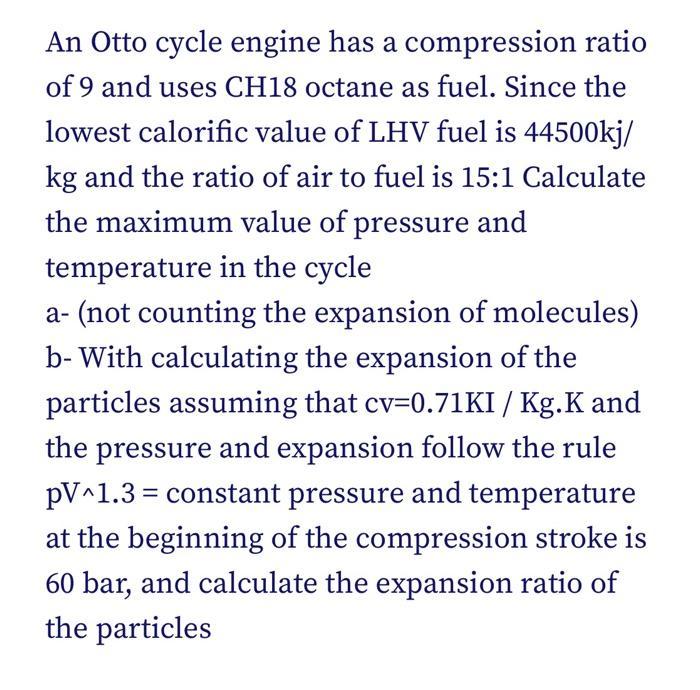
An Otto cycle engine has a compression ratio of 9 and uses CH18 octane as fuel. Since the lowest calorific value of LHV fuel is 44500kj/ kg and the ratio of air to fuel is 15:1 Calculate the maximum value of pressure and temperature in the cycle a- (not counting the expansion of molecules) b- With calculating the expansion of the particles assuming that cv=0.71KI / Kg.K and the pressure and expansion follow the rule pV^1.3= constant pressure and temperature at the beginning of the compression stroke is 60 bar, and calculate the expansion ratio of the particles
Step by Step Solution
3.74 Rating (211 Votes )
There are 3 Steps involved in it
Step: 1
2 C8H18 25 O2 16 CO2 18 H2O From the equation The stoichiometric ratio 2 moles of Octane t...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


