Question: 21. The gas phase reaction A 4B obeys zeroth-order kinetics with r = 0.25 mol dm- hr at 200C. Starting with pure A at
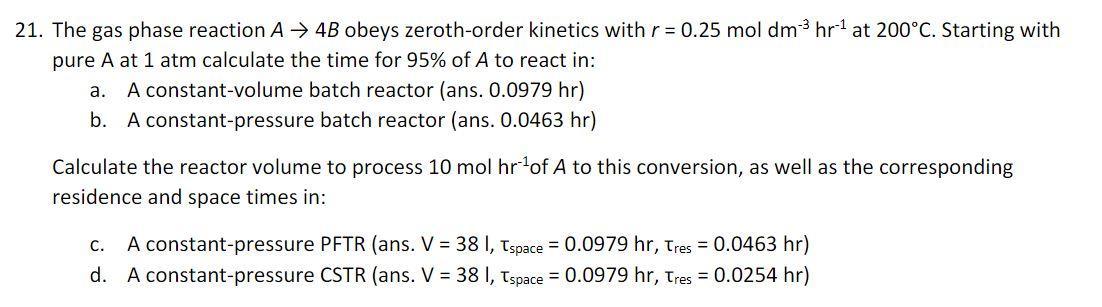
21. The gas phase reaction A 4B obeys zeroth-order kinetics with r = 0.25 mol dm- hr at 200C. Starting with pure A at 1 atm calculate the time for 95% of A to react in: a. A constant-volume batch reactor (ans. 0.0979 hr) b. A constant-pressure batch reactor (ans. 0.0463 hr) Calculate the reactor volume to process 10 mol hrof A to this conversion, as well as the corresponding residence and space times in: c. A constant-pressure PFTR (ans. V = 38 I, Tspace = 0.0979 hr, Tres = 0.0463 hr) CSTR (ans. V = 38 1, Tspace = 0.0979 hr, Tres = 0.0254 hr) d. A constant-pressure
Step by Step Solution
3.59 Rating (153 Votes )
There are 3 Steps involved in it
P T A4B MA we 1 atm 200C Out Law 025 mal PV 1am CA CA... View full answer

Get step-by-step solutions from verified subject matter experts


