Question
9) will the reaction below occur spontaneously. @ 25C if [fe] and [ed *] are 0.6M for Fe 2+ and 0.01m for Cd+ 2+
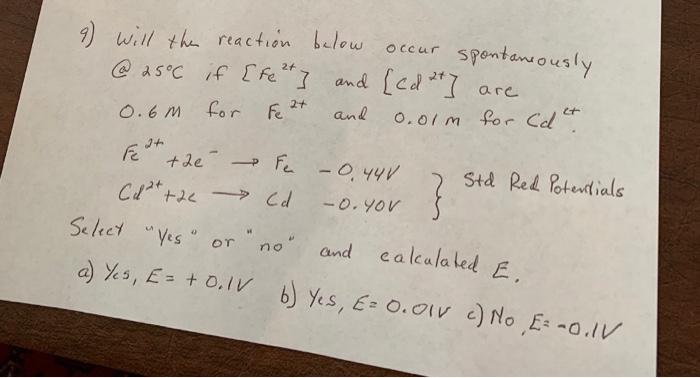
9) will the reaction below occur spontaneously. @ 25C if [fe] and [ed *] are 0.6M for Fe 2+ and 0.01m for Cd+ 2+ Fe+ the Fe -0.44V +2e - Cd+ +2 Cd -0. YOU } Std Red Potentials Select "Yes" or "no" and calculated E. a) Yes, E = + 0.1V b) Yes, E= 0.01 v c) No E= -0.IV
Step by Step Solution
There are 3 Steps involved in it
Step: 1
9 concentration of Fe2 Fe2 06 M Concentration of Cd2 Cd2 001M Standard reducti...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Reporting and Analysis
Authors: Lawrence Revsine, Daniel Collins, Bruce Johnson, Fred Mittelstaedt, Leonard Soffer
7th edition
1259722651, 978-1259722653
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



