Question
A pure sample of solid benzoic acid (CzH6O2) weighing 1.221 g was placed in a constant-volume bomb calorimeter and burned in an oxygen atmosphere.
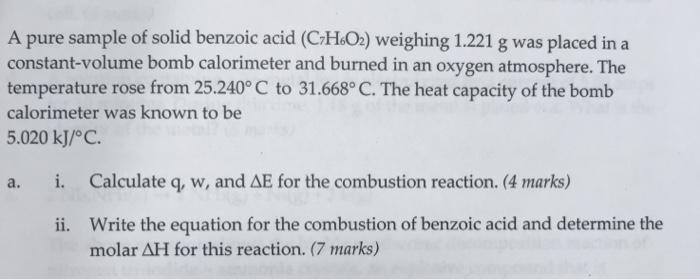
A pure sample of solid benzoic acid (CzH6O2) weighing 1.221 g was placed in a constant-volume bomb calorimeter and burned in an oxygen atmosphere. The temperature rose from 25.240 C to 31.668 C. The heat capacity of the bomb calorimeter was known to be 5.020 kJ/C. a. i. Calculate q, w, and AE for the combustion reaction. (4 marks) ii. Write the equation for the combustion of benzoic acid and determine the molar AH for this reaction. (7 marks)
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
A pure sample of benzoic acid 74602 weiging 1221g was placed in ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Law questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



