Answered step by step
Verified Expert Solution
Question
1 Approved Answer
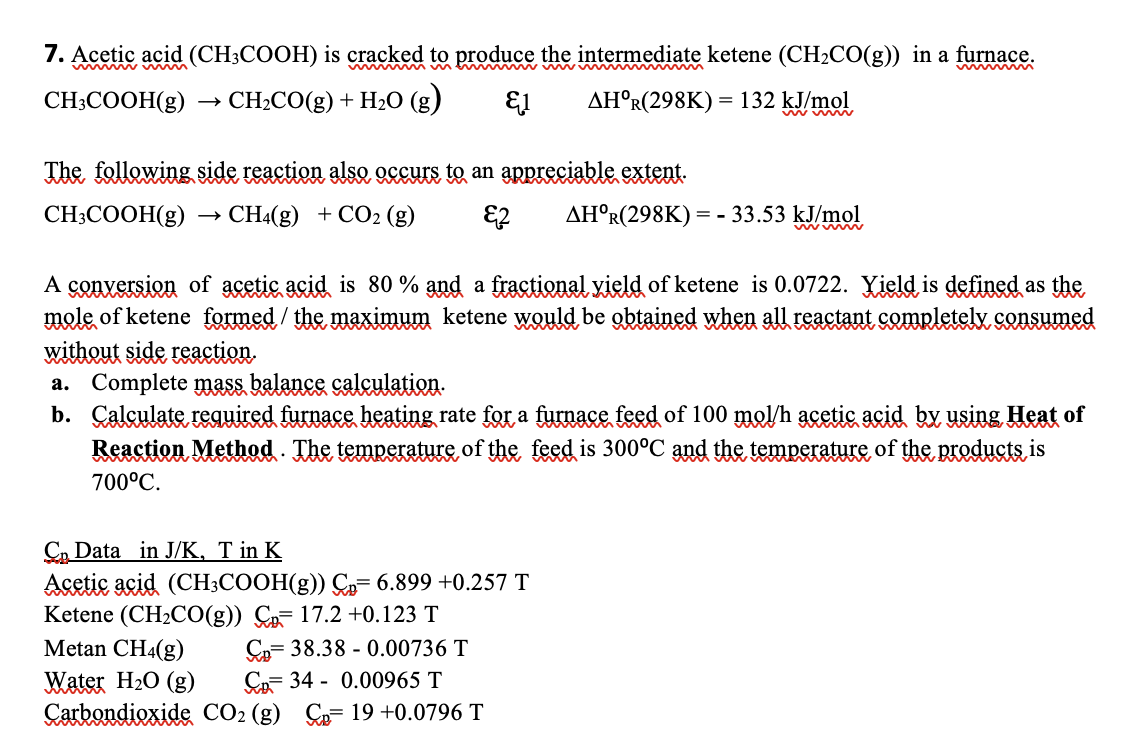
Acetic acid ( C H 3 C O O H ) is cracked to produce the intermediate ketene ( C H 2 C O (
Acetic acid is cracked to produce the intermediate ketene in a furnace.
The following side reaction also occurs to an appreciable extent.
A conversion of acetic acid is and a fractional vield of ketene is Yield is defined as the
mole of ketene formed the maximum ketene would be obtained when all reactant completelv consumed
without side reaction.
a Complete mass balance calculation.
b Calculate required furnace heating rate for a furnace feed of acetic acid by using Heat of
Reaction Method. The temperature of the feed is and the temperature of the products is
Data in in
Acetic acid
Ketene
Metan
Water
Carbondioxide
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


